Define Reaction as a Nucleophile ?
Majority of organic reactions are polar . Flow of electrons from one
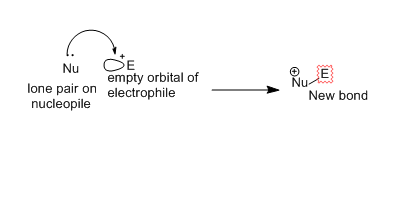
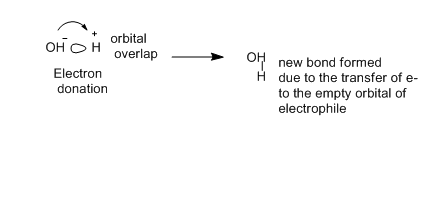
molecule to another during the reaction . The electron donor is known as nucleophile (nucleus loving). while the electron acceptor is called the electrophile (electron-loving). There is a charge attraction is the key to the reactions. The nucleophile likes nuclei because they are positively charged and the electrophile likes electrons because they are negatively
charged. However, reaction occurs due to the flow of electrons from a nucleophile to an empty orbital of electrophile. Electrons are supplied by nucleophiles out of bonds and electrophiles that accept electrons into antibonding orbitals. Electrostatic forces provide a attraction between molecules in chemical reactions. In organic reactions the orbitals of the nucleophile and electrophile are directional.
Step by step
Solved in 3 steps with 3 images









