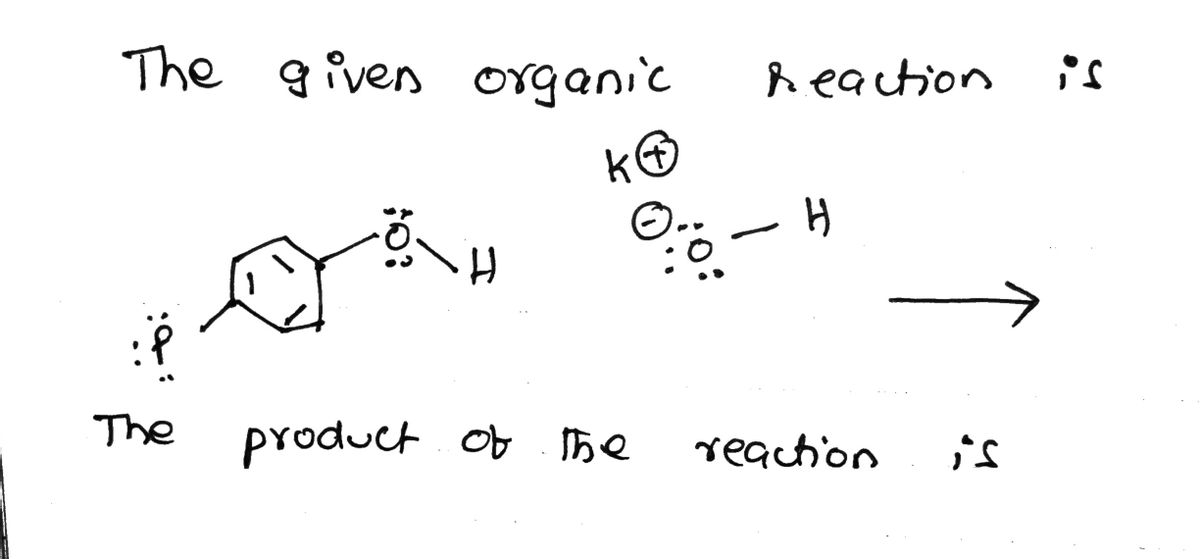
Curved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the products of the following reaction. Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. B 482 KO Select to Draw 133,809 0:0—4 001 7
Curved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the products of the following reaction. Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. B 482 KO Select to Draw 133,809 0:0—4 001 7
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
13
![**Title: Understanding Electron Flow in Organic Reactions**
**Introduction:**
Curved arrows are used to illustrate the flow of electrons in chemical reactions. This technique is crucial for understanding how chemical reactions proceed at the molecular level.
**Instructions:**
Follow the curved arrows in the given diagram to identify the products of the reaction. Ensure to depict all lone pairs and charges as needed. Inorganic byproducts of the reaction should be disregarded for this exercise.
**Diagram Analysis:**
1. **Reactants:**
- A benzene ring is attached to a structure with an oxygen atom bonded to a potassium cation (K⁺).
- The oxygen atom also forms bonds with two hydrogen atoms, and has lone pairs shown.
- The negative charge indicates an ionic bond with potassium (represented as K⁺).
2. **Curved Arrows:**
- The first arrow originates from a lone pair on the oxygen atom and points towards the bond between oxygen and hydrogen, suggesting the initiation of a chemical change.
- The second arrow indicates the breaking of the bond between the hydrogen and its attachment, likely leading to the loss of a hydrogen ion (H⁺) in the reaction.
**Product Prediction:**
Students are expected to draw the resulting molecules using the starting structure and the electron flow indicated by the arrows. The primary focus is on transferring electrons and forming the new structure while maintaining accurate electron counts and charges.
**Conclusion:**
By following the arrows, you identify how electrons are transferred, predicting the new molecular structure after the reaction. This helps in visualizing reaction mechanisms and understanding chemical processes more thoroughly.
Use the space below to draw your predicted product:
[Select to Draw]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F327c4acc-aab0-4600-9811-c42079067cf3%2Fe14f3a8b-d31c-4fcd-ad49-34657abf0fc1%2F40czflb_processed.jpeg&w=3840&q=75)
Transcribed Image Text:**Title: Understanding Electron Flow in Organic Reactions**
**Introduction:**
Curved arrows are used to illustrate the flow of electrons in chemical reactions. This technique is crucial for understanding how chemical reactions proceed at the molecular level.
**Instructions:**
Follow the curved arrows in the given diagram to identify the products of the reaction. Ensure to depict all lone pairs and charges as needed. Inorganic byproducts of the reaction should be disregarded for this exercise.
**Diagram Analysis:**
1. **Reactants:**
- A benzene ring is attached to a structure with an oxygen atom bonded to a potassium cation (K⁺).
- The oxygen atom also forms bonds with two hydrogen atoms, and has lone pairs shown.
- The negative charge indicates an ionic bond with potassium (represented as K⁺).
2. **Curved Arrows:**
- The first arrow originates from a lone pair on the oxygen atom and points towards the bond between oxygen and hydrogen, suggesting the initiation of a chemical change.
- The second arrow indicates the breaking of the bond between the hydrogen and its attachment, likely leading to the loss of a hydrogen ion (H⁺) in the reaction.
**Product Prediction:**
Students are expected to draw the resulting molecules using the starting structure and the electron flow indicated by the arrows. The primary focus is on transferring electrons and forming the new structure while maintaining accurate electron counts and charges.
**Conclusion:**
By following the arrows, you identify how electrons are transferred, predicting the new molecular structure after the reaction. This helps in visualizing reaction mechanisms and understanding chemical processes more thoroughly.
Use the space below to draw your predicted product:
[Select to Draw]
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY