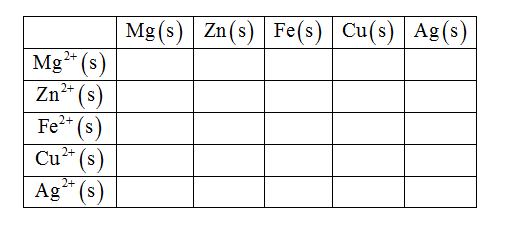
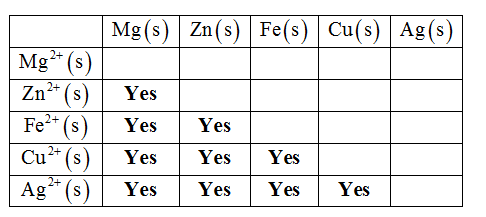
Cu(NO, Ag(s) silver n AgNO Identify whether a reaction occurs between each metal and metal cation in the table. Mg(s) Zu(s) Fe(s) Cu(s) Ag(s) Mg + (aq) Zn+ (aq) Fe (aq) Cu (aq) Cu2+ Ag' (aq) Answer Bank reaction no reaction about us careers privacy policy terms.of use contact us help

The reaction between each metal and metal cation is determined by the electrochemical series of metals. The metals, which are at the top of the series, are more reactive and can reduce the metal ions present at the bottom of the series.
The electrochemical series of metals is shown below.
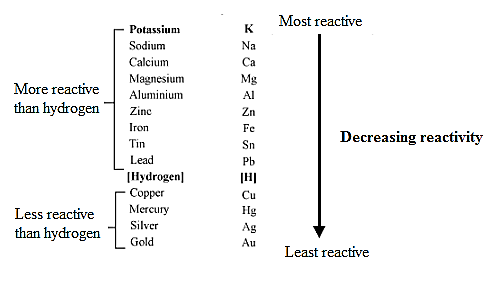
In the above electrochemical series of metals, potassium, sodium, and calcium is above than magnesium. Therefore, only potassium, sodium, and calcium can reduce magnesium ion. Since, the given metals are magnesium, zinc, iron, copper, and silver, so they will not react with magnesium ion.
In the above electrochemical series of metals, magnesium is above than zinc. Therefore, magnesium will reduce the magnesium ion as shown below.
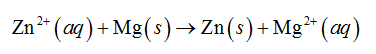
In the above electrochemical series of metals, magnesium and zinc are above than iron. Therefore, magnesium and zinc will reduce the iron ion as shown below.
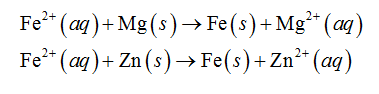
In the above electrochemical series of metals, magnesium, zinc and iron are above than copper. Therefore, magnesium, zinc and iron will reduce the copper ion as shown below.
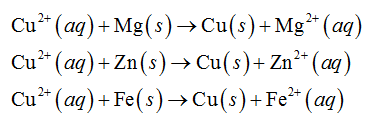
In the above electrochemical series of metals, magnesium, zinc, iron and copper are above than silver. Therefore, magnesium, zinc, iron and copper will reduce the silver ion as shown below.
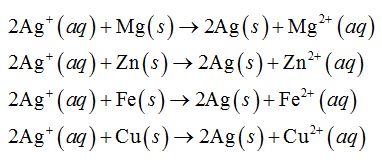
Whether a reaction occurs between each metal and metal cation or not in the Table is identified as shown below.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 8 images









