Consider the reaction A(aq) →→ B(aq) (1) The average reaction rate r can be measured if the concentration of the reagent or the product is known at two different times: r=−Δ[A]/Δt for the reagent, and r=Δ[B]/Δt for the product, where [A] and [B] notate the concentration of A and B in solution, and Δ is always defined as the difference between "final" and "initial": Δ[A]=[A]t2−[A]t1 (t2 and t1 are subscripts) and Δt=t2−t1 (2 and 1 are subscript) A reaction proceeds according to the chemical equation (1). If the initial concentration of B is 0.1053 mol L-1, and after 194.5 s it increases to 0.4275 mol L-1, what is the rate of the reaction in mol L-1 s-1? r=? mol L-1 s-1. (Use scientific notation)
Consider the reaction
A(aq) →→ B(aq) (1)
The average
r=−Δ[A]/Δt for the reagent, and r=Δ[B]/Δt for the product, where [A] and [B] notate the concentration of A and B in solution, and Δ is always defined as the difference between "final" and "initial": Δ[A]=[A]t2−[A]t1 (t2 and t1 are subscripts) and Δt=t2−t1 (2 and 1 are subscript)
A reaction proceeds according to the chemical equation (1). If the initial concentration of B is 0.1053 mol L-1, and after 194.5 s it increases to 0.4275 mol L-1, what is the rate of the reaction in mol L-1 s-1?
r=? mol L-1 s-1. (Use scientific notation)
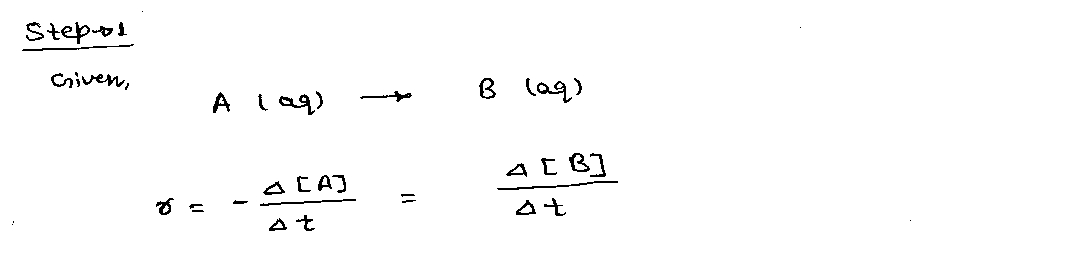
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









