Consider the following balanced equation. 2 Sb (s) + 3 H2O (g) → Sb2O3 (s) + 3 H2 (g) 5.0 L of H2O gas, with a pressure of 1.29 atm and a temperature of 96.7 °C reacted. How many mol of Sb2O3 were formed? Write out the problem on paper showing all conversion factors, unit cancellations, calculations, s.f., etc. Answer the questions related to the setup and calculation for this problem. Be sure to use our periodic table to calculate any molar masses needed (rounded to proper number of decimal places), otherwise your values might be slightly off and answers may be marked as incorrect. Abbreviate units as follows: grams = g, moles = mol, liters = L, atmospheres = atm What temperature (with proper s.f.) is entered into the equation? Enter the value in the first blank and the units in the second blank. Calculate the mol of H2O reacted (enter value only, with proper s.f.). mol Use the three blanks to enter the number, unit, and substance (in this order) that appears in the denominator of the stoichiometry conversion factor. Enter the value for the amount of Sb2O3 formed (in mol). mol
Ideal and Real Gases
Ideal gases obey conditions of the general gas laws under all states of pressure and temperature. Ideal gases are also named perfect gases. The attributes of ideal gases are as follows,
Gas Laws
Gas laws describe the ways in which volume, temperature, pressure, and other conditions correlate when matter is in a gaseous state. The very first observations about the physical properties of gases was made by Robert Boyle in 1662. Later discoveries were made by Charles, Gay-Lussac, Avogadro, and others. Eventually, these observations were combined to produce the ideal gas law.
Gaseous State
It is well known that matter exists in different forms in our surroundings. There are five known states of matter, such as solids, gases, liquids, plasma and Bose-Einstein condensate. The last two are known newly in the recent days. Thus, the detailed forms of matter studied are solids, gases and liquids. The best example of a substance that is present in different states is water. It is solid ice, gaseous vapor or steam and liquid water depending on the temperature and pressure conditions. This is due to the difference in the intermolecular forces and distances. The occurrence of three different phases is due to the difference in the two major forces, the force which tends to tightly hold molecules i.e., forces of attraction and the disruptive forces obtained from the thermal energy of molecules.
Consider the following balanced equation.
2 Sb (s) + 3 H2O (g) → Sb2O3 (s) + 3 H2 (g)
5.0 L of H2O gas, with a pressure of 1.29 atm and a temperature of 96.7 °C reacted. How many mol of Sb2O3 were formed?
Write out the problem on paper showing all conversion factors, unit cancellations, calculations, s.f., etc. Answer the questions related to the setup and calculation for this problem. Be sure to use our periodic table to calculate any molar masses needed (rounded to proper number of decimal places), otherwise your values might be slightly off and answers may be marked as incorrect. Abbreviate units as follows: grams = g, moles = mol, liters = L, atmospheres = atm
What temperature (with proper s.f.) is entered into the equation? Enter the value in the first blank and the units in the second blank.
Calculate the mol of H2O reacted (enter value only, with proper s.f.).
mol
Use the three blanks to enter the number, unit, and substance (in this order) that appears in the denominator of the stoichiometry conversion factor.
Enter the value for the amount of Sb2O3 formed (in mol).
mol
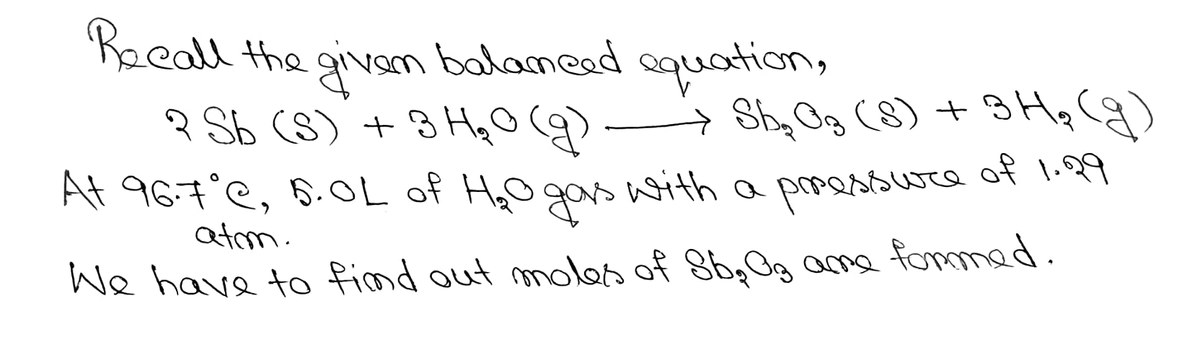
Step by step
Solved in 3 steps with 3 images









