Consider a water molecule (H₂O). The two hydrogen atoms are positively charged, the oxygen atom is doubly negatively charged. The bond angle between the hydrogen atoms is 104.5°. The oxygen-hydrogen distance is 0.096 nm. Ľ 104.5 0.096 nm T:
Consider a water molecule (H₂O). The two hydrogen atoms are positively charged, the oxygen atom is doubly negatively charged. The bond angle between the hydrogen atoms is 104.5°. The oxygen-hydrogen distance is 0.096 nm. Ľ 104.5 0.096 nm T:
Related questions
Question
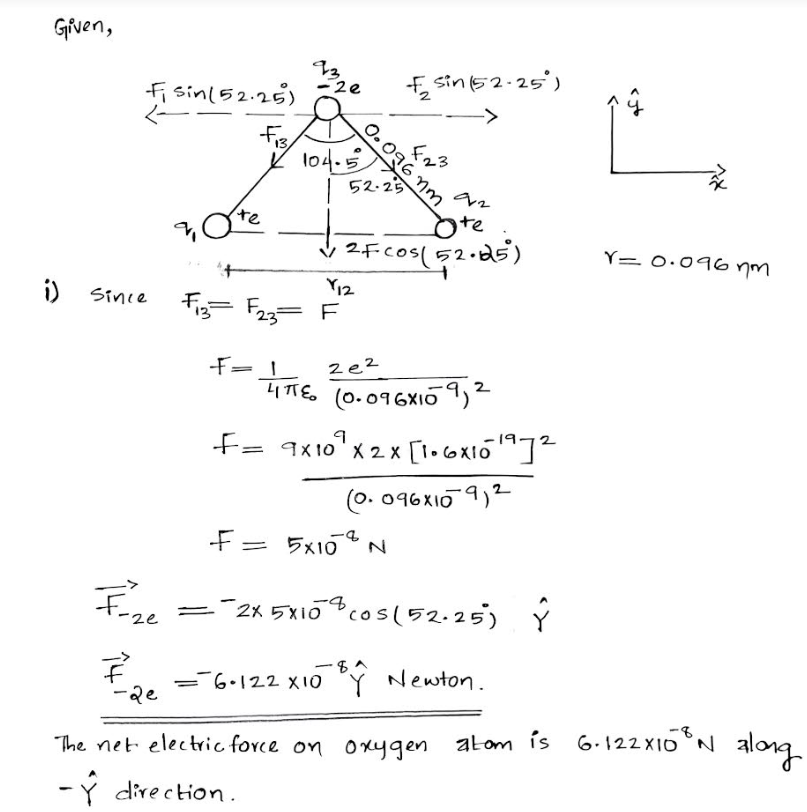
![Consider a water molecule (H₂O). The two hydrogen atoms are positively charged, the oxygen
atom is doubly negatively charged. The bond angle between the hydrogen atoms is 104.5°. The
oxygen-hydrogen distance is 0.096 nm.
104.5"
0.096 nm
T:
Considering the atoms as non-bonded, independent charges... what is the net
electric force vector on the oxygen atom (blue)?
insidering the atoms as non-bonded, independent charges... what is the
potential energy of this arrangement?
Iculate the electric dipole moment (i.e. dipole vector) of the water molecule,
using the axes in the figure to establish unit vectors. Hint: Treat the molecule as two
independent dipoles, and add the moments as vectors.
I claim it is p = -1.88 x 10-299 Cm. Am I correct? Why is it negative?
(NOTE: The measured value of the dipole moment of water is about [p] =
0.617 x 10-29 C m, or about 1/3 of our calculated value.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd1e65d3f-3c77-4e7f-88fc-bf17213b1ce1%2Fcf9fc714-8a60-4582-a75e-d4cf2e21172e%2Fw48vxum_processed.png&w=3840&q=75)
Transcribed Image Text:Consider a water molecule (H₂O). The two hydrogen atoms are positively charged, the oxygen
atom is doubly negatively charged. The bond angle between the hydrogen atoms is 104.5°. The
oxygen-hydrogen distance is 0.096 nm.
104.5"
0.096 nm
T:
Considering the atoms as non-bonded, independent charges... what is the net
electric force vector on the oxygen atom (blue)?
insidering the atoms as non-bonded, independent charges... what is the
potential energy of this arrangement?
Iculate the electric dipole moment (i.e. dipole vector) of the water molecule,
using the axes in the figure to establish unit vectors. Hint: Treat the molecule as two
independent dipoles, and add the moments as vectors.
I claim it is p = -1.88 x 10-299 Cm. Am I correct? Why is it negative?
(NOTE: The measured value of the dipole moment of water is about [p] =
0.617 x 10-29 C m, or about 1/3 of our calculated value.

Transcribed Image Text:Now the water molecule is placed in region IV- that is, outside of the outer cylinder from part b
in this test. The angle between the molecule's axis of symmetry and the vector to the molecule
is 20°, as shown below. The molecule is 2 cm from the outside face of the outer cylinder. Use
the measured value of the dipole moment in the following sections (see NOTE above). DO NOT
USE the value you calculated in part e.iii.
i.
ii.
iii.
2 cm
20⁰
What is the torque on the water molecule due to the electric field from the
cylinders? Hint: Consider the result from part b.
/hat is the potential energy of the dipole in the arrangement shown above?
If the dipole were free to rotate, but not translate, what is the minimum possible
potential energy of the dipole?
Expert Solution
Step 1
"Since you have asked multiple question, I am answering first question as per Bartleby guidelines. The underlined values are are the solutions of the question asked."
Step by step
Solved in 3 steps with 3 images
