Complete the ex przenian fer the YaH Of apperance of prodects 3 T rare Of disa perrance of reactents in each of T FOllaing rxh's 1) Add eitner a zero or a positiv or negativetnteger toealn anfucr ned ##aFot bary 204ig)→ 200 cg) Arc07 At NO2 191+ NOz19) ANg05 At ALNO,I AING 17 At 3) 2INO La) → In la) ) → Iq 19)+ 2Nota) AINU] A/NO At At
Please show how to solve
![Complete the ex przenian fer the
YaH Of apperance of prodects
3
T rare Of disa perrance of reactents
in each of T FOllaing rxh's
1) Add eitner a zero or a positiv
or negativetnteger toealn
anfucr ned ##aFot bary
204ig)→ 200 cg)
Arc07
At
NO2 191+
NOz19)
ANg05
At
ALNO,I
AING
17
At
3) 2INO La) → In la) )
→ Iq 19)+ 2Nota)
AINU]
A/NO
At
At](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ffef23454-38d1-4713-a68e-ac868e92b8e3%2Ffae704df-d2d1-45a4-be39-d8eec1c354ef%2F8v6n8ok.jpeg&w=3840&q=75)
We’ll answer the first question since the exact one wasn’t specified. Please submit a new question specifying the one you’d like answered.
(1)
Here the given reaction is
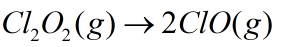
For which the rate of disappearance reactant and rate of appearance of product is shown as:
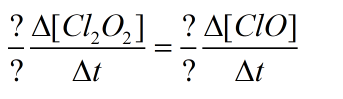
To fill the above blank fields, it is important to understand what is rate of reaction for appearance of product and disappearance of reactants.
Actually, in a given reaction, initially the product concentration is zero. As the reaction proceed, the rate of disappearance of reactants become equal to rate of appearance of products. Hence, the rate of disappearance of reactants have negative (-) sign and rate of appearance of products have (+)ve sign in the rate expression.
Also, when the rate of reaction is written, it is considered as the rate of change of concentration of product or reactant with respect to time. While the stoichiometric coefficients of the reactants or products are written in the denominator of rate expression as follows:
For example, in a reaction like:
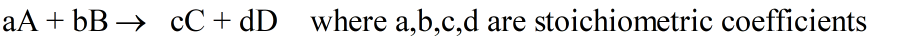
The rate of appearance of product and disappearance of reactants is written as:
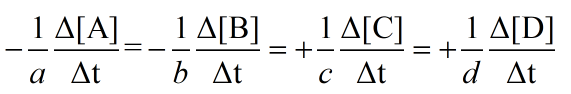
Step by step
Solved in 2 steps with 6 images









