Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
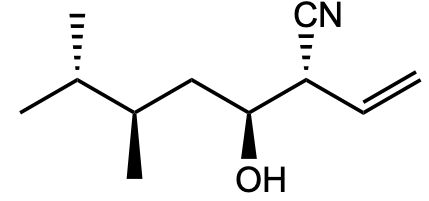
how many stereoisomers are here?

Transcribed Image Text:The image depicts the structural formula of a chemical compound. This compound includes several key features:
1. **Carbon Chain**: The structure shows a carbon chain backbone, denoting a linear arrangement of carbon atoms connected by single bonds.
2. **Functional Groups**:
- **Hydroxyl Group (OH)**: Located on a middle carbon, represented by an oxygen and hydrogen atom bonded together. This group is characteristic of alcohols and contributes to the molecule's polarity and potential reactivity with acids.
- **Nitrile Group (CN)**: Comprised of a carbon triple-bonded to a nitrogen atom, represented by "CN". This is indicative of a nitrile group, which can impact the compound's solubility and reactivity.
- **Alkene (Double Bond)**: Present near the right end of the molecule, represented by a double line between two carbon atoms, indicating a double bond.
3. **Stereochemistry**:
- The compound illustrates stereochemistry with wedges and dashed lines showing the three-dimensional orientation of bonds. Solid wedges indicate bonds coming out of the plane towards the viewer, while dashed lines represent bonds going behind the plane. This is important in determining the molecule's stereoisomers and properties.
This structural formula emphasizes the significance of functional groups and stereochemistry in organic compounds, affecting their chemical behavior and interactions.
Expert Solution
Step 1
The molecule given is,
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY