CI -D А - НзО+ B- H9SO4/H3O* C- 1. Li/pentane 2. D20 D- H2/Pt E- Li/NH3 F- 1. BH3/THF 2. H2O2/OH-
CI -D А - НзО+ B- H9SO4/H3O* C- 1. Li/pentane 2. D20 D- H2/Pt E- Li/NH3 F- 1. BH3/THF 2. H2O2/OH-
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Select the correct reagent/s for carrying out each of the following reactions shown below. Put a letter in each box.

Transcribed Image Text:This image provides a visual representation of chemical reactions with specific reagents and their resulting products.
1. **Top Reaction**:
- **Reactant**: A terminal alkyne (1-butyne).
- **Product**: A ketone (butanone).
- **Reagent Options**: The reagents likely used in this reaction are option B – HgSO₄/H₃O⁺. This indicates an oxymercuration-demercuration process that converts the alkyne to a ketone.
2. **Middle Reaction**:
- **Reactant**: A phenyl-substituted terminal alkyne (phenylacetylene).
- **Product**: A conjugated diene (styrene).
- **Reagent Options**: The conversion suggests the use of option E – Li/NH₃, which typically results in the partial reduction of alkynes to trans-alkenes.
3. **Bottom Reaction**:
- **Reactant**: A chlorocyclohexane.
- **Product**: A deuterated cycloalkane (cyclohexane-d).
- **Reagent Options**: Reagents used here are likely C – 1. Li/pentane, 2. D₂O. This process involves the reduction of the alkyl halide using lithium and the introduction of deuterium through D₂O.
**Reagent Key**:
- **A**: H₃O⁺
- **B**: HgSO₄/H₃O⁺
- **C**: 1. Li/pentane 2. D₂O
- **D**: H₂/Pt
- **E**: Li/NH₃
- **F**: 1. BH₃/THF 2. H₂O₂/OH⁻
These reactions highlight various chemical transformations involving reduction, hydration, and deuteration processes using specific reagents.
Expert Solution
Step 1
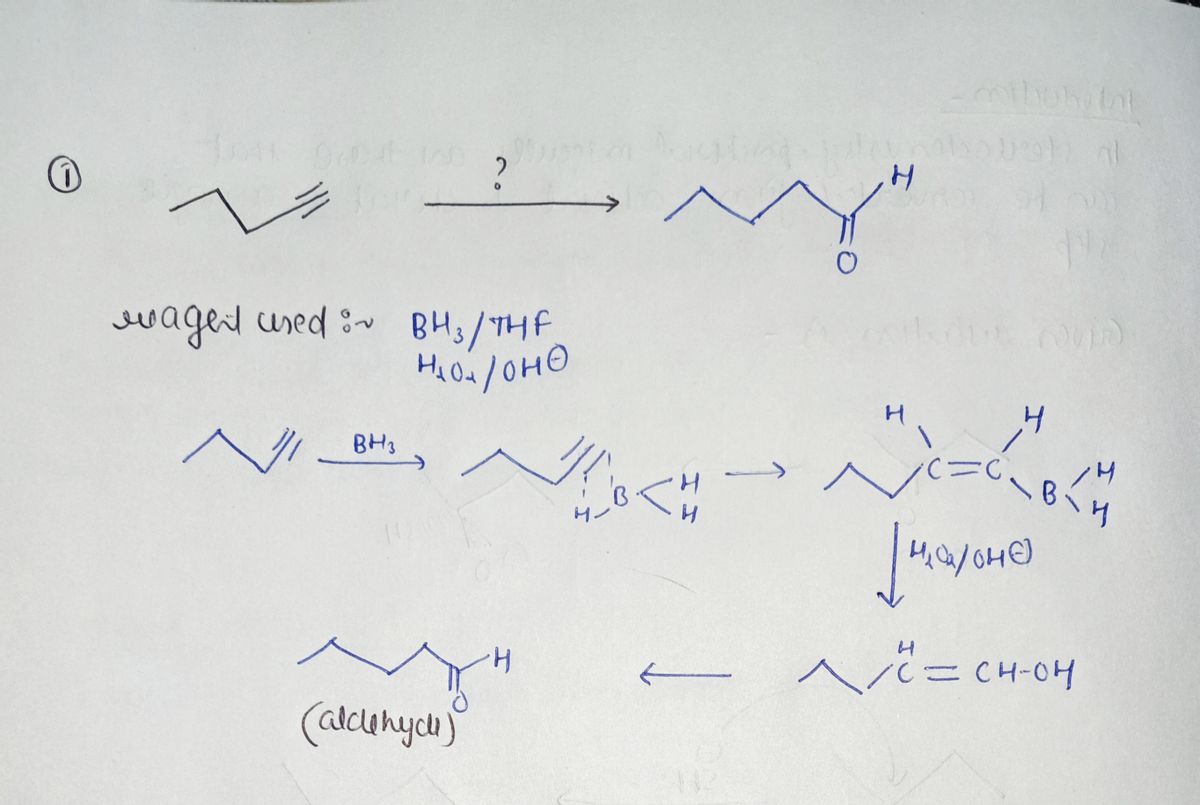
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The