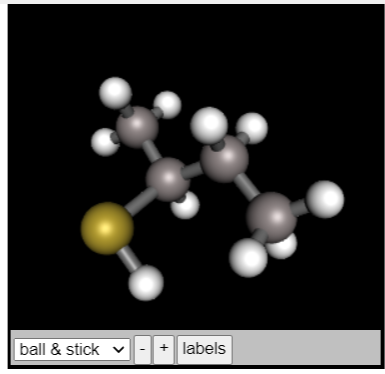
Choose all possible representations of the molecule shown in the 3D window from the list below. Be sure that any three-dimensional representation has the same configuration as the molecule in the window. ball & stick v + labels SH SH CH3 CH2CH3 SH Previous Next CH3
Choose all possible representations of the molecule shown in the 3D window from the list below. Be sure that any three-dimensional representation has the same configuration as the molecule in the window. ball & stick v + labels SH SH CH3 CH2CH3 SH Previous Next CH3
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
check all that apply

Transcribed Image Text:**Title: Exploring Molecular Structures**
**Instruction:**
Choose all possible representations of the molecule shown in the 3D window from the list below. Be sure that any three-dimensional representation has the same configuration as the molecule in the window.
**3D Molecular Model:**
- The display is a ball-and-stick model.
- Different colors represent different atoms. A yellow sphere might indicate a sulfur atom, while gray and white spheres are typically carbon and hydrogen atoms respectively.
**Representation Options:**
1. **Option 1:**
- A linear structural formula showing a simple lineup of atoms.
2. **Option 2:**
- A tetrahedral arrangement indicating a specific 3-dimensional configuration. It features a central carbon bonded to various groups, including two SH groups and ethyl groups.
3. **Option 3:**
- Another linear structural formula, similar in layout to Option 1 but indicating possibly different substituents.
**Navigation:**
- Use the "Previous" or "Next" buttons to move through different screens or questions.
**Analysis:**
To correctly select the representations, look for the spatial arrangement and connectivity of atoms in the 3D model and compare with the 2D structural formulas provided.

Transcribed Image Text:### Molecular Structure and Identification
#### 3D Model
The image shows a 3D ball-and-stick model of a molecular structure. The atoms are represented by spheres with varying colors to indicate different elements. Bonds between atoms are shown as sticks connecting the spheres.
- **Ball-and-Stick Model**: This representation helps in visualizing the geometry of the molecule and the spatial arrangement of its atoms.
- **Gray Spheres**: Typically represent carbon atoms.
- **White Spheres**: Typically represent hydrogen atoms.
- **Yellow Sphere**: Likely represents a sulfur atom.
#### Structural Formulas
Below the 3D model are several structural formulas. These are possible representations of the molecular structure depicted above. The boxes next to the structures are for selecting the structure that corresponds to the 3D model.
1. **Structure 1**:
- SH group attached to a linear carbon chain.
2. **Structure 2**:
- SH group bonded to a chiral center, with a branched carbon chain (isopropyl group) attached.
3. **Structure 3**:
- SH group attached to a straightforward linear carbon chain.
4. **Structure 4**:
- Chiral center with SH, CH3, and CH2CH3 as substituents.
5. **None of the Above**: An option if none of the structures match the 3D model.
### Explanation of Options
- The given options provide different structural isomers or configurations that might represent the molecule shown in the 3D model.
- The goal is to match the 3D structure with its correct 2D representation by identifying the spatial arrangement of atoms.
#### Learning Objectives
- Recognize and interpret different molecular representations.
- Understand spatial molecular geometry using 3D visualization.
- Identify correct structural formulas based on 3D models.
Expert Solution
Step 1
Given,
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY