
Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for you. To get remaining sub-part solved please repost the complete question and mention the sub-parts to be solved..
Isotopes are the atoms of an element that differ in number of neutrons present in them. As mass number is the sum of neutrons and protons, isotopes have different mass numbers.
Radioactive isotopes are those which decay spontaneously releasing alpha particles, beta particles or gamma rays etc.
Half-life is the time taken for amount of a radioactive isotope to drop to 50% of its initial amount.
Radioactive decay follows first order kinetics.
So, half-life (t1/2) is related to rate constant (k) as t1/2 = 0.693/k.
The integrated rate law for first order kinetics is as shown below:
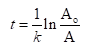
Here, t is the time of progress of reaction, k is the rate constant, Ao is the initial amount of radioisotope and A is the amount of radioisotope left after time t.
Step by step
Solved in 4 steps with 6 images









