
During electrochemical change or known as "cell" the various quantities are interrelated with each other through some defined equations. These in turn are dependent on the electrons transferred in that redox change.
Given:
The temperature is 25 degree Celsius.
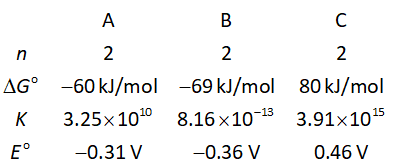
The formula to calculate ΔGᴼ is shown below.
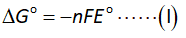
Here,
The standard Gibbs energy change is “ΔGᴼ”.
The number of electrons transferred is “n”.
The Faraday’s constant is “F”.
The cell potential is “Eᴼ”.
The value of Faraday’s constant is 96500 C/mol.
The formula to calculate equilibrium constant is shown below.
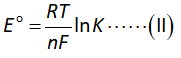
Here,
The temperature is “T”.
The gas constant is “R”.
The value of gas constant is 8.314 J/K-mol.
The conversion of temperature in Celsius to Kelvin is done below.
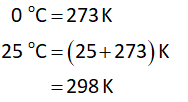
For cell A:
Suppose the value of cell potential is correct.
Thus, the value of Eᴼ is -0.31 V.
Substitute the value of cell potential in equation (I).
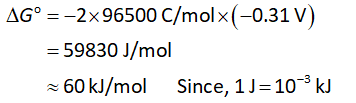
Substitute the value of cell potential in equation (II).
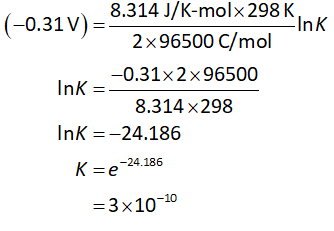
Thus, the calculated value of ΔGᴼ and K are wrong. But only one value out of Eᴼ, ΔGᴼ and K can be incorrect.
Hence, the value of Eᴼ is incorrect.
Step by step
Solved in 6 steps with 12 images









