Please ignore my work! Not sure if it is correct!
Please find the molarity of NaOH solution for each trial?
Thanks

The ratio of moles of solute to the volume of solution in liters is known as molarity.
Oxalic acid exists in dehydrated form
The formula of dehydrated oxalic acid (C2O4H2).2H2O
The molar mass of dehydrated oxalic acid = (90 +36) gmol-1
= 126 gmol-1
1 mol of oxalic acid requires 2 moles of NaOH for neutralization.
In case of first trial;
Mass of oxalic acid = 0.306 g
Volume of NaOH = 20.63 mL
The calculation of moles is shown below:
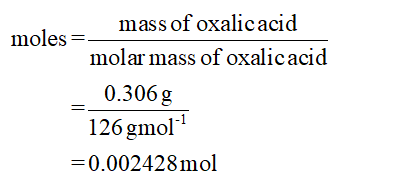
0.002428 mol of oxalic acid = 2 ×0.002428 mol of NaOH
= 0.004856 mol of NaOH
The calculation of molarity is shown below:
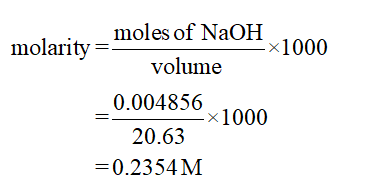
For trial 2:
Mass of oxalic acid = 0.303 g
Volume of NaOH = 20.25 mL
The calculation of moles is shown below:
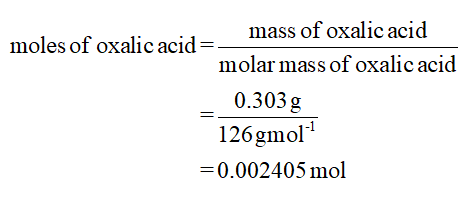
0.002405 mol of oxalic acid = 2× 0.002405 mol of NaOH
= 0.00481 mol of NaOH
Step by step
Solved in 9 steps with 7 images









