Calculations Using the Mole Learning Goal: 2.32x1023 13C atoms To learn how to convert grams to moles and to use the mole to find the number of atoms in a sample. Submit Previous Answers The mole (abbreviated mol) is a counting unit used to simplify calculations that would otherwise involve very large numbers. The mole is equivalent to the number of carbon atoms in exactly 12 g of isotopically pure 6.02 x 1023. This number is known as Avogadro's number in honor of Amedeo Avogadro. 12 C, or v Correct Avogadro's number can be used as a conversion factor between moles and atoms as shown here: Part C 6.02x1023 atoms Based on your answer in Part B, how many electrons are in this amount of 13 C? 1 mole of atoms Express your answer numerically in electrons. The molar mass of a substance is the mass of one mole • View Available Hint(s) of a substance and is written in units of grams per mole. The molar mass of an atom is equivalent to its atomic mass whereas the molar mass of a substance is equivalent to its formula weight. x" |X| x•10" electrons
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
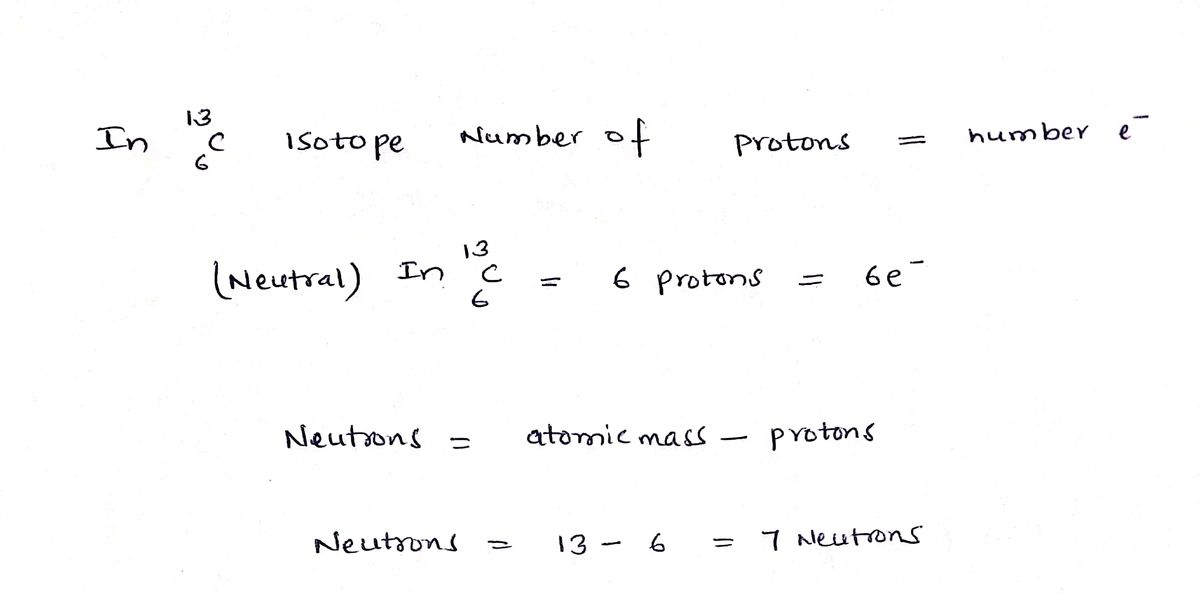
Part C: Based on your answer in Part B, how many electrons are in this amount of 13C?
Part D: Based on your answer in Part B, how many neutrons are in this amount of 13C?

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









