Calculate the bubble point temperature for a liquid hydrocarbon mix
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
type solution in word

Transcribed Image Text:Calculate the bubble point temperature for a liquid hydrocarbon mix of 30% n-pentane
and 70% n-heptane (use Antoine equation to calculate vapor pressures), for a system at
pressure of 3.0 atmospheres. Also, calculate the vapor mole fractions in equilibrium
with this liquid mixture.
B
log 10 p* = A-
T+C
Compound
Formula
Range (°C)
A
B
C
n-Pentane
n-C5H12
13.3 to 36.8
6.84471
1060.793
231.541
000010
n-Heptane
n-C7H16
25.9 to 99.3
6.90253 1267.828 216.823
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 6 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
can you show me how to do this in excel? i think it needs goalseek and will look like this kind of?
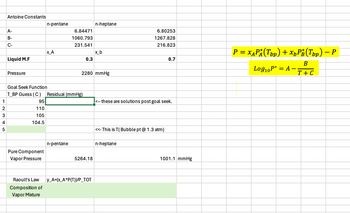
Transcribed Image Text:Antoine Constants
n-pentane
n-heptane
A-
B-
C-
6.84471
1060.793
6.80253
1267.828
231.541
216.823
Х_А
x b
Liquid M.F
0.3
0.7
Pressure
2280 mmHg
Goal Seek Function
T_BP Guess (C)
Residual (mmHg)
95
these are solutions post goal seek.
110
105
12345
104.5
Pure Component
Vapor Pressure
n-pentane
5264.18
<<This is T(Bubble pt @ 1.3 atm)
n-heptane
Raoult's Law
Composition of
Vapor Mixture
y_A=(x A*P(T))/P_TOT
1001.1 mmHg
-
P = x^P (Top) + x¸³¿(Top) − P
bp
Log10 P* = A
B
T + C
bp
Solution
Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The