synthesis for this

The Grignard reagent is denoted by the formula R-MgX, where R is an alkyl or aryl group and X is a halogen. The key feature of this reagent is its high nucleophilicity. Grignard reagents can undergo nucleophilic addition on the carbonyl compounds to furnish the corresponding alcohol. The tertiary alkyl halides can be synthesized from the corresponding tertiary alcohols by treating them with the concentrated acids.
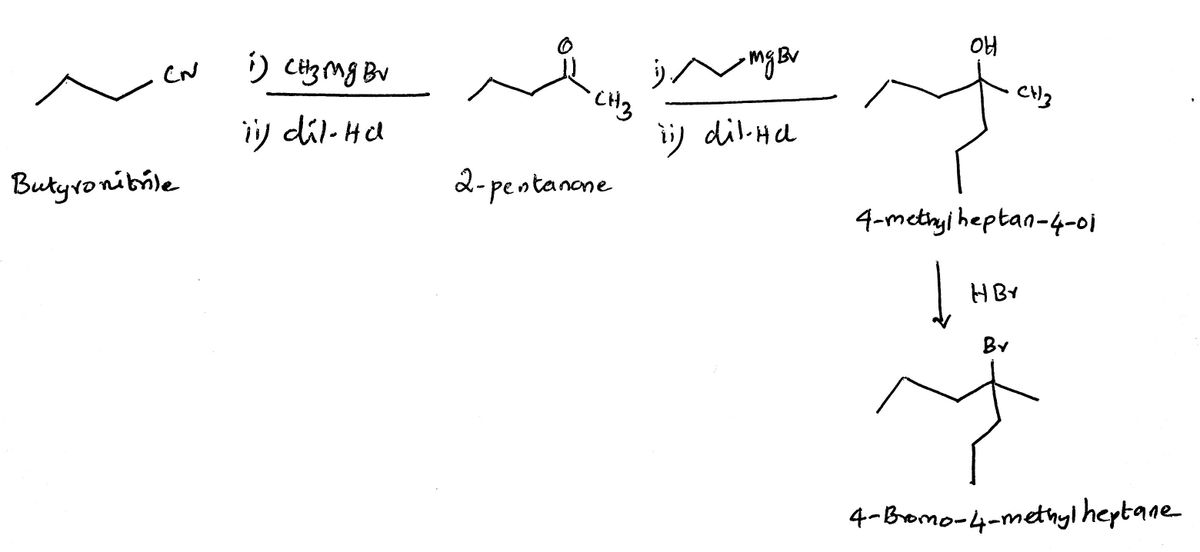
The reaction of butyronitrile with methyl magnesium bromide followed by the hydrolysis produces 2-pentanone. The ketone on further reaction with propyl magnesium bromide followed by the hydrolysis yields the tertiary alcohol, 4-methylheptan-4-ol. On reaction with HBr, the tertiary alcohol yields the corresponding tertiary alkyl bromide, 4-bromo-4-methylheptane via the formation of the tertiary carbocation. The scheme is provided below:
Step by step
Solved in 3 steps with 1 images









