Use curved arrows to show the movement of electrons in each equation.

The movement of electrons in each equation should be shown using the curved arrows:
The given equations are as follows,
Curved arrows are used to show the flow of electrons in the organic reactions.
Curved arrows are represented as follows,
In this notation, the tail of a curved arrow represents electron donor species and head of curved arrow represents electron acceptor species.
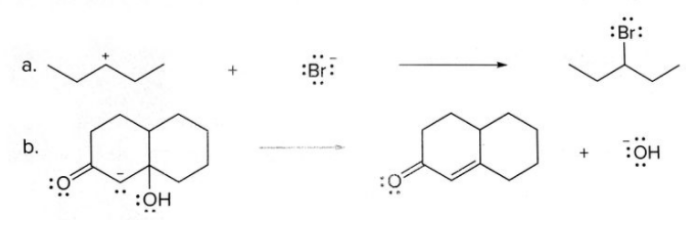
The given equation (a) is :
In this equation, Bromine has a negative charge on it, so, is electron rich species and carbon having positive charge is electron deficient species. In this reaction, bromine donates its electron to the electropositive carbon and forms a carbon - bromine bond.
Thus, the curved arrow for the equation (a) can be represented as given below :
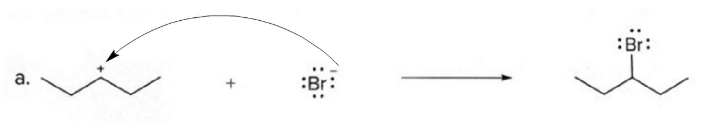
Step by step
Solved in 5 steps with 8 images









