Which is the most stable: 3,4-dimethyl-2-hexene, 2,3-dimethyl-2-hexene, or 4,5-dimethyl-2-hexene? a. Which compound has the largest heat of hydrogenation? b. Which compound has the smallest heat of hydrogenation?

The stability of an alkene increases as the number of alkyl substituents bonded to its sp2 carbon increases. In second alkene (2,3-dimethyl-2-hexene) three methyl group is attached to the sp2 carbon atom. In the first alkene two alkyl groups are attached to its sp2 carbon atom.
In the third alkene there is no alkyl group were attached.
Therefore, 2,3-dimethyl-2-hexene is the most stable alkene and its structure is,
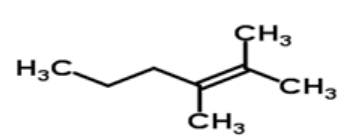
The stability of an alkene increase as the number of alkyl substituents bonded to its sp2 carbon increases. In second alkene (2,3-dimethyl-2-hexene) three methyl group is
attached to the sp2 carbon atom. In the first alkene two alkyl groups are attached to its sp2 carbon atom. In the third alkene (4,5-dimethyl-2-hexene) there is no alkyl group
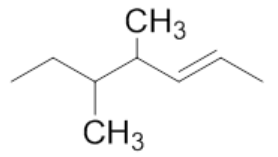
were attached. Therefore, 4,5-dimethyl-2-hexene is the least stable alkene. The least stable
alkene has the largest heat of hydrogenation.
Step by step
Solved in 3 steps with 3 images









