Blackboard X - с 1 Gmail < Esc app.101edu.co YouTube STARTING AMOUNT Aktiv Chen X 14°C Mostly cloudy Maps X b Search res x Welcome to MyTCC Jill F3 Your Sets X 403 Forbidden: Ac... b Answered: x ADD FACTOR 1.01 How many moles of propylene (CH) are in 25.0 1 0 4.15 x 10- 25.0 molecules Cale Quiz 3-Fl x Question 31 of 50 QL 12.01 6.022 x 102 b Answered: x ANSWER 1 36.03 mol CH g C₂H/mol 9 42.09 ASUS сда of the substance? 0.694 g C₂H b Answered: x FIO RESET 2 0.594 F G The oxidat x + Q☆ PH Sc W Insert
Blackboard X - с 1 Gmail < Esc app.101edu.co YouTube STARTING AMOUNT Aktiv Chen X 14°C Mostly cloudy Maps X b Search res x Welcome to MyTCC Jill F3 Your Sets X 403 Forbidden: Ac... b Answered: x ADD FACTOR 1.01 How many moles of propylene (CH) are in 25.0 1 0 4.15 x 10- 25.0 molecules Cale Quiz 3-Fl x Question 31 of 50 QL 12.01 6.022 x 102 b Answered: x ANSWER 1 36.03 mol CH g C₂H/mol 9 42.09 ASUS сда of the substance? 0.694 g C₂H b Answered: x FIO RESET 2 0.594 F G The oxidat x + Q☆ PH Sc W Insert
Computer Networking: A Top-Down Approach (7th Edition)
7th Edition
ISBN:9780133594140
Author:James Kurose, Keith Ross
Publisher:James Kurose, Keith Ross
Chapter1: Computer Networks And The Internet
Section: Chapter Questions
Problem R1RQ: What is the difference between a host and an end system? List several different types of end...
Related questions
Question
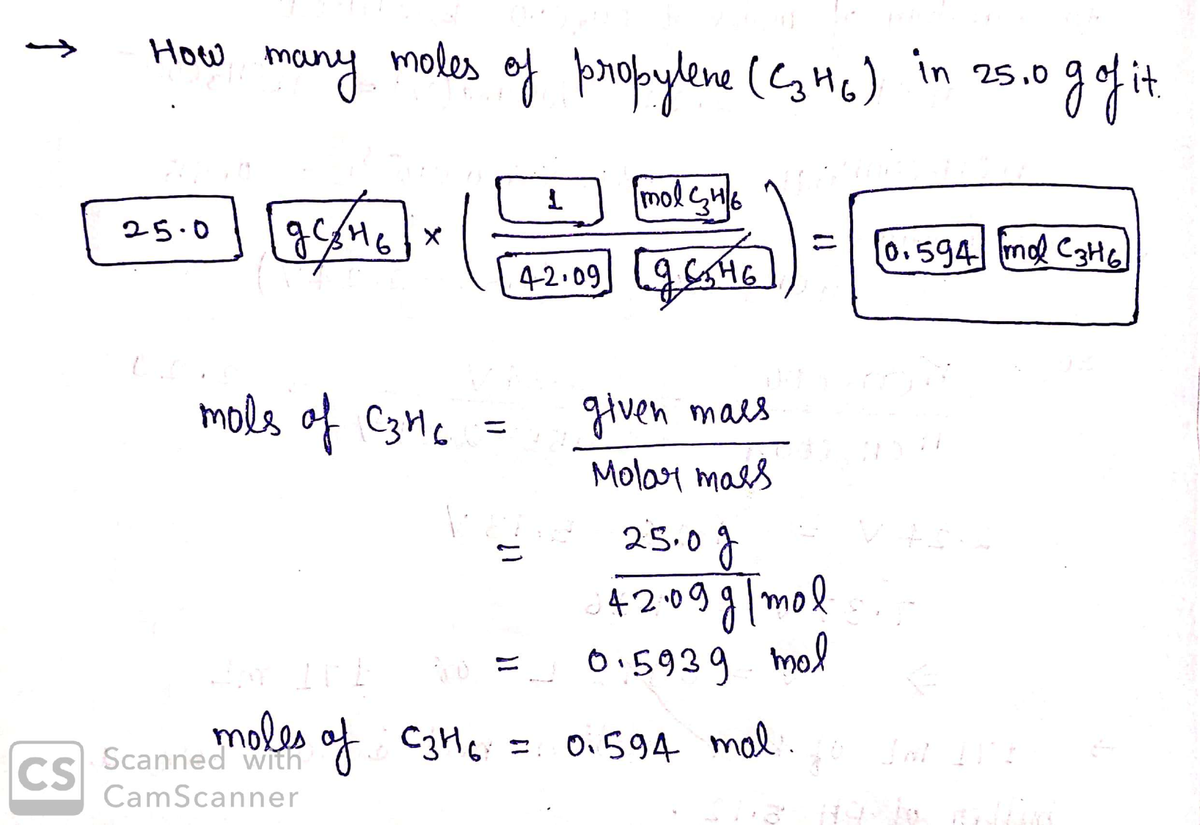
![### Propylene Mole Calculation Example
#### Question 31 of 50
**Prompt:**
How many moles of propylene (C₃H₆) are in 25.0 g of the substance?
#### Calculation Process:
1. **Starting Amount:**
The weight of the substance is provided as 25.0 grams.
2. **Conversion Setup:**
To find the number of moles, you will use the molecular weight of propylene along with the given mass.
3. **Formula:**
\[
\text{Number of moles} = \frac{\text{given mass (g)}}{\text{molar mass (g/mol)}}
\]
#### Reference Data and Buttons:
- **Molecular Weights and Constants:**
- Hydrogen (H): 1.01
- Carbon (C): 12.01
- Molar Mass of C₃H₆: 42.09 g/mol
- Avogadro's Number: 6.022 x 10²³
- **Calculation Interface:**
- Input fields are provided to enter values for the starting amount and conversion factors.
- Various selectable options for units such as molecules C₃H₆, mol C₃H₆, g C₃H₆/mol, and g C₃H₆ are available.
#### Final Steps:
- Use the provided molecular weight for propylene (42.09 g/mol) to compute the moles in 25.0 grams.
- Ensure to use correct units and significant figures in your calculations.
**Note:** This setup is typically used in educational environments to teach students how to convert grams of a substance into moles using molecular weight.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F0406c5f8-494f-428b-ae59-e78691671853%2Fabaf3e51-f34e-4598-a932-3199641aa47e%2Fao7o40u_processed.jpeg&w=3840&q=75)
Transcribed Image Text:### Propylene Mole Calculation Example
#### Question 31 of 50
**Prompt:**
How many moles of propylene (C₃H₆) are in 25.0 g of the substance?
#### Calculation Process:
1. **Starting Amount:**
The weight of the substance is provided as 25.0 grams.
2. **Conversion Setup:**
To find the number of moles, you will use the molecular weight of propylene along with the given mass.
3. **Formula:**
\[
\text{Number of moles} = \frac{\text{given mass (g)}}{\text{molar mass (g/mol)}}
\]
#### Reference Data and Buttons:
- **Molecular Weights and Constants:**
- Hydrogen (H): 1.01
- Carbon (C): 12.01
- Molar Mass of C₃H₆: 42.09 g/mol
- Avogadro's Number: 6.022 x 10²³
- **Calculation Interface:**
- Input fields are provided to enter values for the starting amount and conversion factors.
- Various selectable options for units such as molecules C₃H₆, mol C₃H₆, g C₃H₆/mol, and g C₃H₆ are available.
#### Final Steps:
- Use the provided molecular weight for propylene (42.09 g/mol) to compute the moles in 25.0 grams.
- Ensure to use correct units and significant figures in your calculations.
**Note:** This setup is typically used in educational environments to teach students how to convert grams of a substance into moles using molecular weight.
Expert Solution
Step 1
:: Solution::
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Computer Networking: A Top-Down Approach (7th Edi…
Computer Engineering
ISBN:
9780133594140
Author:
James Kurose, Keith Ross
Publisher:
PEARSON

Computer Organization and Design MIPS Edition, Fi…
Computer Engineering
ISBN:
9780124077263
Author:
David A. Patterson, John L. Hennessy
Publisher:
Elsevier Science

Network+ Guide to Networks (MindTap Course List)
Computer Engineering
ISBN:
9781337569330
Author:
Jill West, Tamara Dean, Jean Andrews
Publisher:
Cengage Learning

Computer Networking: A Top-Down Approach (7th Edi…
Computer Engineering
ISBN:
9780133594140
Author:
James Kurose, Keith Ross
Publisher:
PEARSON

Computer Organization and Design MIPS Edition, Fi…
Computer Engineering
ISBN:
9780124077263
Author:
David A. Patterson, John L. Hennessy
Publisher:
Elsevier Science

Network+ Guide to Networks (MindTap Course List)
Computer Engineering
ISBN:
9781337569330
Author:
Jill West, Tamara Dean, Jean Andrews
Publisher:
Cengage Learning

Concepts of Database Management
Computer Engineering
ISBN:
9781337093422
Author:
Joy L. Starks, Philip J. Pratt, Mary Z. Last
Publisher:
Cengage Learning

Prelude to Programming
Computer Engineering
ISBN:
9780133750423
Author:
VENIT, Stewart
Publisher:
Pearson Education

Sc Business Data Communications and Networking, T…
Computer Engineering
ISBN:
9781119368830
Author:
FITZGERALD
Publisher:
WILEY