please answer part two (Please answer "What is BP of the solvent, and the authors state... why is that important"). thank you
"The 3-Sulfolene is the prime molecule used for synthesis for the 1,3-butadiene molecule. The mechanism involves breaking of two C-S bonds in 3-sulfolene to generate 1,3-butadiene in the presence of heat. The reaction can be shown as below:
This reaction is a type of pericyclic reaction that involves breaking and making of bonds in a single step and hence there will be no intermediate formed. The solvent used here is water." https://www.bartleby.com/questions-and-answers/3-sulfolene-a-butadiene-source-for-a-diels-alde-synthesis.-please-be-prepared-to-take-a-quiz-on-this/8bf8358d-dbdf-46f9-97db-6f744d66c0cd


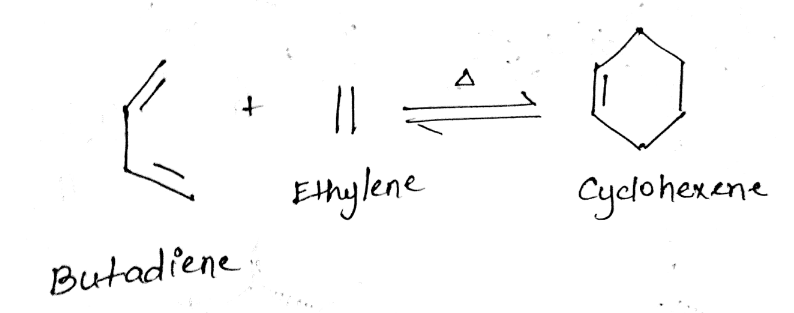
Diels-Alder reaction is a type of pericyclic reaction between a conjugated diene (two double bonds) and a dienophile (an alkene with an electron-withdrawing group).
The simplest Diels-Alder reaction is the reaction of butadiene with ethene to cyclohexane.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images









