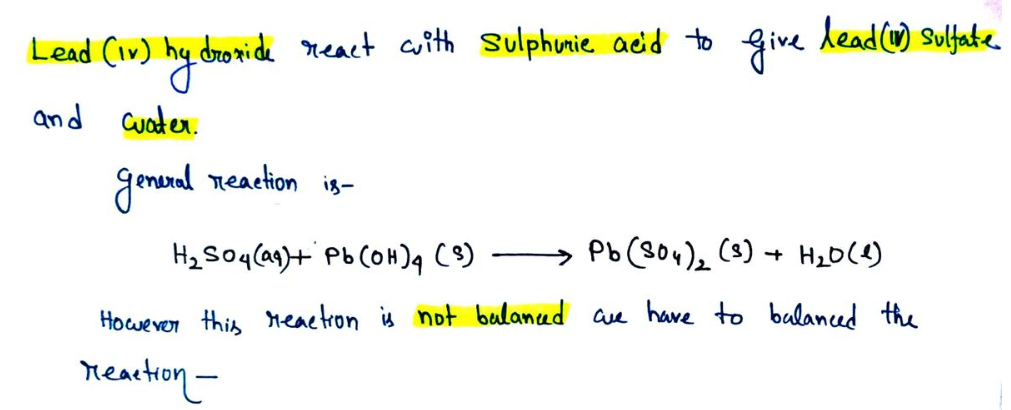
Balance the following chemical equation (if necessary): H₂SO4(aq) + Pb(OH)4(S) → Pb(SO4)2(S) + H₂O(1)
Balance the following chemical equation (if necessary): H₂SO4(aq) + Pb(OH)4(S) → Pb(SO4)2(S) + H₂O(1)
Computer Networking: A Top-Down Approach (7th Edition)
7th Edition
ISBN:9780133594140
Author:James Kurose, Keith Ross
Publisher:James Kurose, Keith Ross
Chapter1: Computer Networks And The Internet
Section: Chapter Questions
Problem R1RQ: What is the difference between a host and an end system? List several different types of end...
Related questions
Question
![**Balance the Chemical Equation**
**Question 29 of 50:**
Balance the following chemical equation (if necessary):
\[ \text{H}_2\text{SO}_4(aq) + \text{Pb(OH)}_4(s) \rightarrow \text{Pb(SO}_4)_2(s) + \text{H}_2\text{O}(l) \]
**Instructions:**
Use the provided interface to balance the chemical equation. Select coefficients and place them appropriately to ensure the number of atoms of each element is equal on both sides of the equation. You can use the buttons to add or adjust coefficients, states of matter, and compounds.
**Interface Elements:**
- Coefficient Buttons: Numbers 1 through 9 to adjust the coefficients.
- State Symbols: (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous.
- Compounds: Options to add or remove Pb(OH), H\(_2\)O, PbSO\(_4\), H\(_2\)SO\(_4\).
- Reset Button: Resets the entire equation to start over.
- Delete Button: Removes the last entry made.
**Aim:** Balance each element correctly and ensure the equation is stable through proper coefficient selection.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F0406c5f8-494f-428b-ae59-e78691671853%2F0c37e7d9-c881-4d99-8b93-94ef07f497be%2Fmxwfp1_processed.jpeg&w=3840&q=75)
Transcribed Image Text:**Balance the Chemical Equation**
**Question 29 of 50:**
Balance the following chemical equation (if necessary):
\[ \text{H}_2\text{SO}_4(aq) + \text{Pb(OH)}_4(s) \rightarrow \text{Pb(SO}_4)_2(s) + \text{H}_2\text{O}(l) \]
**Instructions:**
Use the provided interface to balance the chemical equation. Select coefficients and place them appropriately to ensure the number of atoms of each element is equal on both sides of the equation. You can use the buttons to add or adjust coefficients, states of matter, and compounds.
**Interface Elements:**
- Coefficient Buttons: Numbers 1 through 9 to adjust the coefficients.
- State Symbols: (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous.
- Compounds: Options to add or remove Pb(OH), H\(_2\)O, PbSO\(_4\), H\(_2\)SO\(_4\).
- Reset Button: Resets the entire equation to start over.
- Delete Button: Removes the last entry made.
**Aim:** Balance each element correctly and ensure the equation is stable through proper coefficient selection.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 4 images

Recommended textbooks for you

Computer Networking: A Top-Down Approach (7th Edi…
Computer Engineering
ISBN:
9780133594140
Author:
James Kurose, Keith Ross
Publisher:
PEARSON

Computer Organization and Design MIPS Edition, Fi…
Computer Engineering
ISBN:
9780124077263
Author:
David A. Patterson, John L. Hennessy
Publisher:
Elsevier Science

Network+ Guide to Networks (MindTap Course List)
Computer Engineering
ISBN:
9781337569330
Author:
Jill West, Tamara Dean, Jean Andrews
Publisher:
Cengage Learning

Computer Networking: A Top-Down Approach (7th Edi…
Computer Engineering
ISBN:
9780133594140
Author:
James Kurose, Keith Ross
Publisher:
PEARSON

Computer Organization and Design MIPS Edition, Fi…
Computer Engineering
ISBN:
9780124077263
Author:
David A. Patterson, John L. Hennessy
Publisher:
Elsevier Science

Network+ Guide to Networks (MindTap Course List)
Computer Engineering
ISBN:
9781337569330
Author:
Jill West, Tamara Dean, Jean Andrews
Publisher:
Cengage Learning

Concepts of Database Management
Computer Engineering
ISBN:
9781337093422
Author:
Joy L. Starks, Philip J. Pratt, Mary Z. Last
Publisher:
Cengage Learning

Prelude to Programming
Computer Engineering
ISBN:
9780133750423
Author:
VENIT, Stewart
Publisher:
Pearson Education

Sc Business Data Communications and Networking, T…
Computer Engineering
ISBN:
9781119368830
Author:
FITZGERALD
Publisher:
WILEY