Atomic mass of a metal can be linked to its specific heat capacity in J/goC units by a simple relation shown below. Determining the specific heat capacity of an unknown metal by calorimetry can help identify the metal based on this relation. Relationship known as Dulong-Petit Law: atomic weight x specific heat capacity = 25 A student was given an unknown metal to be identified. He weighed the metal block and it was 50.0 g. Using a constant temperature bath, he heated the metal to an initial temperature of 70.4 oC. In a calorimeter, he took
1)
Relationship known as Dulong-Petit Law: atomic weight x specific heat capacity = 25
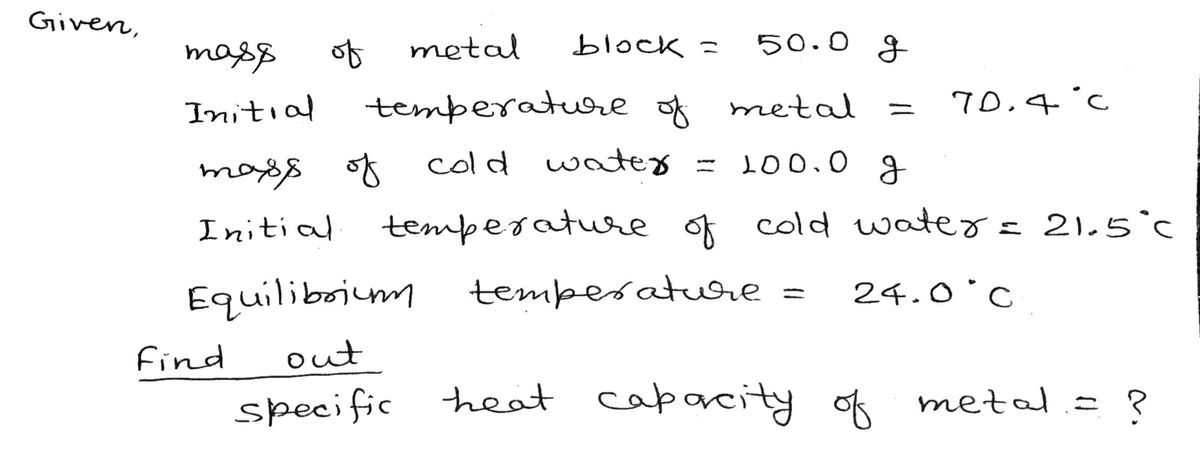
A student was given an unknown metal to be identified. He weighed the metal block and it was 50.0 g. Using a constant temperature bath, he heated the metal to an initial temperature of 70.4 oC. In a calorimeter, he took 100.0 g of cold water and at an initial temperature of 21.5 oC. The hot unknown metal was dropped carefully in the cold water in the calorimeter and the resulting final temperature was measured as 24.0 oC.Calculate the specific heat capacity of the unknown metal. Using the relationship above estimate atomic mass of the unknown metal and use periodic table to identify the unknown metal. [specific heat capacity of water is 4.18 J/goC]
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









