Q: An ideal monatomic gas contracts in an isobaric process from 1.05 m³ to 0.540 m3 at a constant…
A:
Q: An ideal monatomic gas expands isothermally from 0.520 m3 to 1.25 m at a constant temperature of 620…
A: Given data: The initial volume is V1=0.52 m3. The final volume is V2=1.25 m3. Temperature is T=620…
Q: 3 moles of an ideal monatomic gas, initially at 300o K, are expanded from .2 m3 to .5 m3 using an…
A: Given: Moles in gas = 3. Initial temperature of gas (Ti) = 300 K Initial volume of gas (Vi) = 0.2…
Q: Consider 1.00 mol of a monatomic ideal gas for which cv=3/2 R. The gas is subjected to a…
A: Apply Heat euqation. Q =U+W
Q: A monatomic ideal gas initially fills a V0 = 0.45 m3 container at P0 = 85 kPa. The gas undergoes an…
A: Heat absorbed Q3=-W3=-nRT0lnVfVi=-nRP0V0nRlnV1V0=-P0V0lnV1V0
Q: One liter of a gas with y = 1.30 is at 375 Kand 1.32 atm pressure. It is suddenly compressed…
A: given: γ=1.30 Gas of volume, V1 = 1L at initial temperature, T1 = 375 K with initial pressure, P1 =…
Q: A monatomic ideal gas with volume 0.170 L is rapidly compressed, so the process can be considered…
A: The initial temperature of the gas is, T1=3×102 K The final temperature of the gas is, T2=476 K The…
Q: Consider 1.00 mol of a monatomic ideal gas for which cv=3/2 R. The gas is subjected to a…
A:
Q: A cylinder contains 2.00 mol of an ideal monoatomic gas initially at pressure and temperature of…
A:
Q: A 2.00-mol sample of a diatomic ideal gas expands slowly and adiabatically from a pressure of 5.08…
A: Given data- Mole n=2, diatomic ideal gas expands slowly and adiabatically from initial pressure…
Q: An ideal diatomic gas contracts in an isobaric process from 1.15 m to 0.540 m at a constant pressure…
A:
Q: 0.5000 moles of an ideal gas are expanded isothermally and reversibly from 10.0 liters to 20.0…
A: See below
Q: n ideal gas with energy E = NKBT is subjected to a cyclic, quasi-static (i he figure. What is the…
A: An ideal gas with energy E = 52NkBT is subjected to a cyclic, quasi-static (i.e. reversible) process…
Q: ideal monatomic gas (n = 0.001 mol) has an isothermal expansion from 0.1 m3 to 0.2 m3 at 133 degrees…
A:
Q: An ideal gas initially at 345 K undergoes an isobaric expansion at 2.50 kPa. The volume increases…
A:
Q: A monatomic ideal gas with volume 0.195 L is rapidly compressed, so the process can be considered…
A:
Q: A 2.00-mol sample of a diatomic ideal gas expands slowly and adiabatically from a pressure of 4.90…
A:
Q: Exactly one mole of a monatomic perfect gas, originally at 35.0 atm and 25.0°C, expands reversibly…
A:
Q: One mole of an ideal gas initially at a temperature of Ti = 7.4°C undergoes an expansion at a…
A: An ideal gas is at an initial temperature of 7.4 degree-Celcius goes a constant pressure or isobaric…
Q: a) Calculate the net work done by the gas. kJ (b) Calculate the energy added to the gas by heat. kJ…
A: “Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: As shown in the figure, a container with a moveable piston and containing a monatomic ideal gas in…
A: GIVEN the gas volume in the initial state = 7.00 L, pressure of the gas = 3.00 atm, the…
Q: A monatomic ideal gas with volume 0.195 L is rapidly compressed, so the process can be considered…
A:
Q: The temperature of 2.71 moles of a monatomic ideal gas is 295 K. The internal energy of this gas is…
A: Number of moles of mono-atomic ideal gas is n=2.71 mol Temperature is T=295 K Internal energy of gas…
Q: One mole of an ideal gas initially at a temperature of T₁ = 1.4°C undergoes an expansion at a…
A:
Q: A monatomic ideal gas initially fills a V0 = 0.45 m3 container at P0 = 85 kPa. The gas undergoes an…
A: Basic Details The amount of work done in the isobaric expansion can be given as the product of…
Q: Ideal diatomic gas (y = 1.40 ) has initial temperature of 181 K , initial volume of 0.163 m³ , and…
A: Here given the initial pressure 33 kpa. Initial volume 0.163 m³. Final volume 0.338 m³. We have to…
Q: 1.00 mole of an ideal monatomic gas is in a rigid container with a constant volume of 2.00 L. The…
A:
Q: An ideal monatomic gas initially has a temperature of 276 K and a pressure of 7.98 atm. It is to…
A: Given Initial temperature T1 = 276 K Initial pressure P1 = 7.98 atm Initial volume V1 = 426…
Q: In an isothermal process, one mole of an ideal monatomic gas at a temperature T is taken from an…
A:
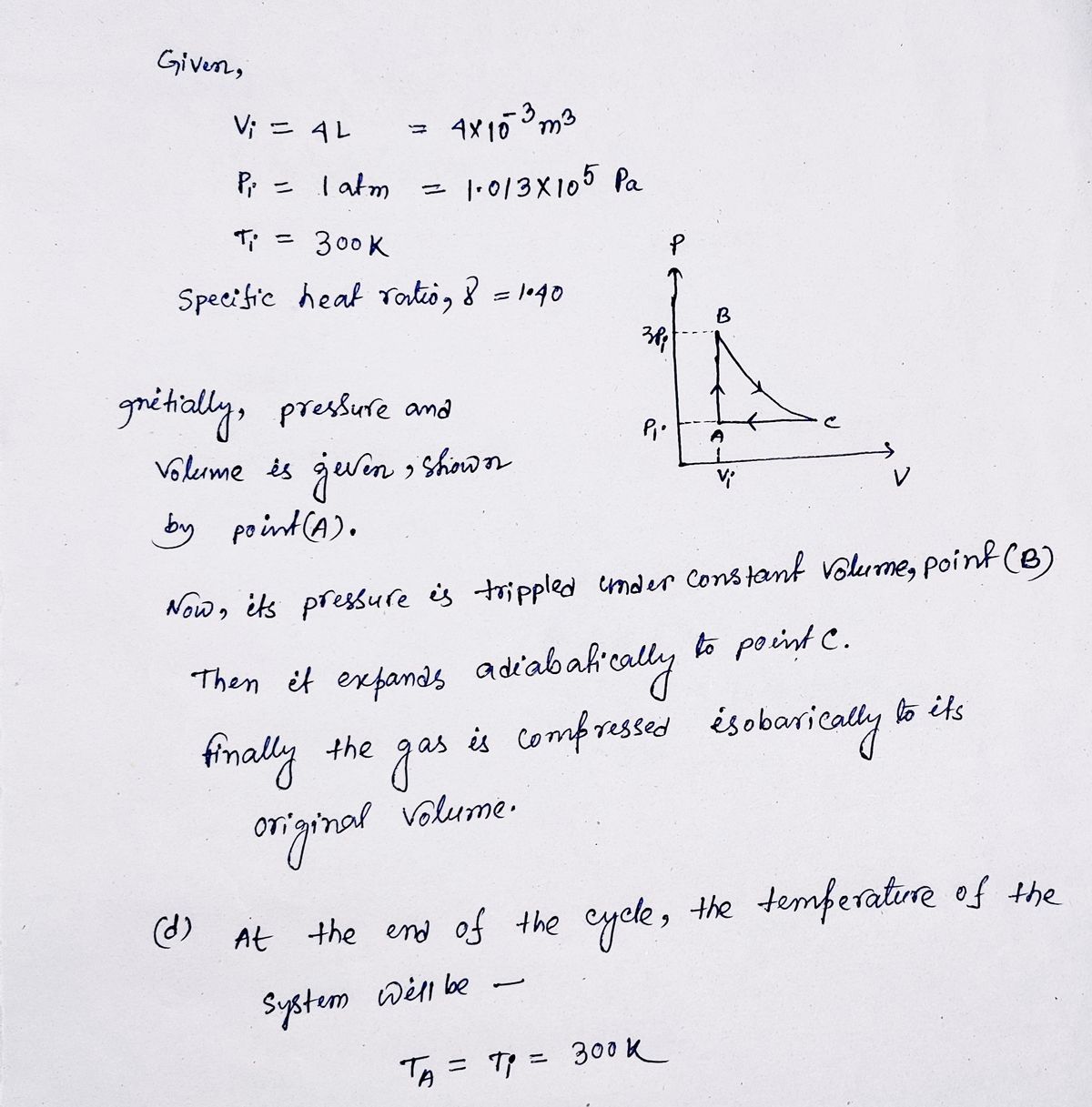
A 4.00 L sample of a diatomic ideal gas with specific heat ratio 1.40, confined to a cylinder, is carried through a closed cycle. The gas is initially at 1.00 atm and at 300 K. First, its pressure is tripled under constant volume. Then, it expands adiabatically to its original pressure. Finally, the gas is compressed isobarically to its original volume.
(d) Find the temperature at the end of the cycle.
K
(e) What was the net work done on the gas for this cycle?
J
Step by step
Solved in 5 steps with 5 images

- As a 3.00-mol sample of a monatomic ideal gas expands adiabatically, the work done on it is −2.50 103 J. The initial temperature and pressure of the gas are 400 K and 2.60 atm. Calculate the following. (a) the final temperature K(b) the final pressure atmA 3-mole of a monatomic ideal gas undergoes an isothermal expansion at 450 K, as the volume increased from 0.010 m³ to 0.060 m³. What is the work done by the gas and the change in the internal energy of the gas respectively during this process? (R = 8.31 J/mol · K)In a constant-volume process, 208 J of energy is transferred by heat to 1.07 mol of an ideal monatomic gas initially at 303 K. (a) Find the work done on the gas. (b) Find the increase in internal energy of the gas. (c) Find its final temperature. K
- A 2.00 mol sample of an ideal diatomic gas at a pressure of 1.10 atm and temperature of 420 K undergoes a process in which its pressure increases linearly with temperature. The final temperature and pressure are 720 K and 1.70 atm . Fid the change in internal energy, work done by the gas, and heat added.A 1 mol sample of a diatomic ideal gas (γ=1.4) expands slowly and adiabatically from a pressure of 18 atm and a volume of 3 L to a final volume of 18 L. What is the final temprature (in K) of the gas? ( Answer no decimal )A monatomic ideal gas initially fills a V0 = 0.45 m3 container at P0 = 85 kPa. The gas undergoes an isobaric expansion to V1 = 1.4 m3. Next it undergoes an isovolumetric cooling to its initial temperature T0. Finally it undergoes an isothermal compression to its initial pressure and volume. 1 Calculate the work done by the gas, W1, in kilojoules, during the isobaric expansion (first process). 2 Calculate the heat absorbed Q1, in kilojoules, during the isobaric expansion (first process). 3 Write an expression for the change in internal energy, ΔU1 during the isobaric expansion (first process). 4 Calculate the work done by the gas, W2, in kilojoules, during the isovolumetric cooling (second process). 5 Calculate the heat absorbed Q2, in kilojoules, during the isovolumetric cooling (second process). 6 Calculate the change in internal energy by the gas, ΔU2, in kilojoules, during the isovolumetric cooling (second process). 7 Calculate the work done by the gas, W3, in kilojoules,…
- In an engine, 0.25 mol of an ideal monatomic gas in the cylinder expands rapidly and adiabatically against the piston. In the process, the temperature of the gas drops from 1150 K to 400 K. How much work does the gas do?a) Consider a process involving an ideal diatomic gas with n = 3mol, following p = aV, where a = 1 x 105 Pa/m³ is a constant. The gas ex- pands from volume V; = 1 m³ to V; = 4m3. P2 Find the (i) work done on the gas. (ii) heat entering the gas. 1 (iii) change in the internal energy of the gas. b) Now consider the cycle depicted in the figure, involving the same amount of gas as in the previous part. A → B is the process described in the previous subtask, B → C an isochor and C → A an isobar. Additionally, V2/V1 = n = 4 and Vi = 1 m³. Find the Pi 3 i) work done by the gas during one loop of the cycle. V1 V2 V ii) thermal efficiency of the cycle. iii) maximum theoretical efficiency of a Car- not cycle having the same temperature extrema as in this cycle. iv) coefficient of performance of the cycle, if it were used as a refrigerator .One mole of an ideal gas, for which CV,m = 3/2R, initially at 298 K and 1.00 × 10^5 Pa undergoes a reversible adiabatic compression. At the end of the process, the pressure is 1.00 × 10^6 Pa. Calculate the final temperature of the gas. Calculate q, w, ΔU, and ΔH for this process.