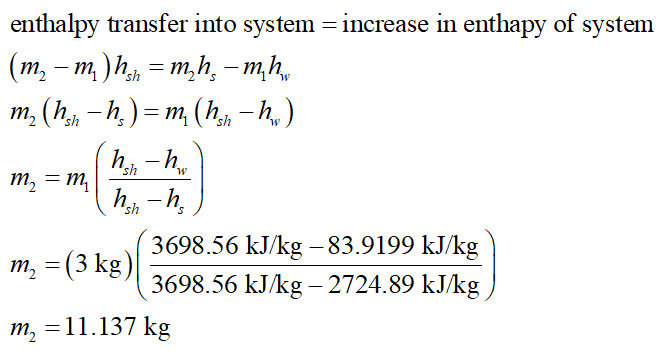
An insulated, vertical piston–cylinder device is purchased from a manufacturer. Upon purchase, it contains 3 kg of water at precisely 20 degrees celcius. The mass of the piston is such that it maintains a constant pressure of 300 kPa inside the cylinder. Liquid water at 2 MPA and 873.15 Kelvin is allowed to enter the cylinder from a supply line until the instant that the last drop of liquid in the cylinder vaporizes (saturated vapor). Find the mass of the steam that has entered [kg].
An insulated, vertical piston–cylinder device is purchased from a manufacturer. Upon purchase, it contains 3 kg of water at precisely 20 degrees celcius. The mass of the piston is such that it maintains a constant pressure of 300 kPa inside the cylinder. Liquid water at 2 MPA and 873.15 Kelvin is allowed to enter the cylinder from a supply line until the instant that the last drop of liquid in the cylinder vaporizes (saturated vapor).
Find the mass of the steam that has entered [kg].
From the steam table, the specific enthalpy of the saturated water at 200C is 83.9199 kJ/kg.
From the steam table, the specific enthalpy of the saturated steam at 300 kPa is 2724.89 kJ/kg.
From the steam table, the specific enthalpy of the superheated steam at 1000 kPa and 6000C is 3698.56 kJ/kg.
Write the energy balance equation and substitute the required values.
Step by step
Solved in 3 steps with 2 images









