An exothermic reaction of reactant (A) forms the economically important product (B) and sell product (C),: AB+C
An exothermic reaction of reactant (A) forms the economically important product (B) and sell product (C),: AB+C
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
plz use excel

Transcribed Image Text:An exothermic reaction of reactant (A) forms the economically important product (B) and sellable by-
product (C),:
Which was carried out isothermally and the following data was recorded:
XA 0
mol
-Am²3-min 1.2
1 (dm³.min)
-TA
mol
FAQ (dm³)
-TA
0.83
333.33
mol
minute
Feed Flow Rate is 100
0.1
1.8
0.56
0.2
1.8
A → B+C
0.56
222.22 222.22
0.3
3
0.33
0.5
3
0.33
0.65
1.2
0.83
0.8
0.75
1.33
0.95
0.5464
1.83
133.33 133.33 333.33 533.33 732.06
of pure A
Q: Determine the reactor volumes in dm³ of a PFR with a conversion of X₁ = 20% followed by a CSTR
that reaches conversion X₂ 20% at the end of a 2 reactor process
Expert Solution
Step 1: The Design equations of a CSTR and PFR and the Levenspiel plot
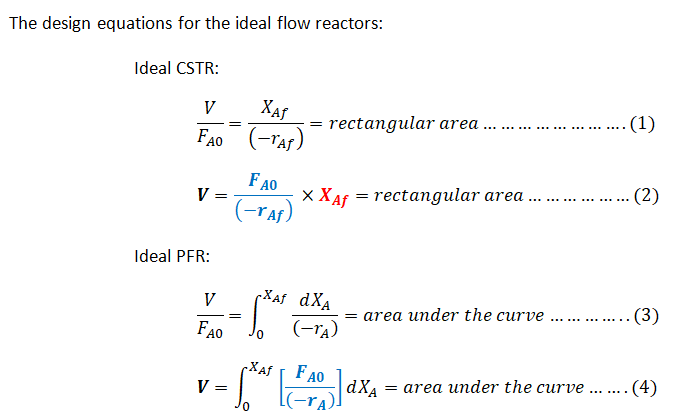
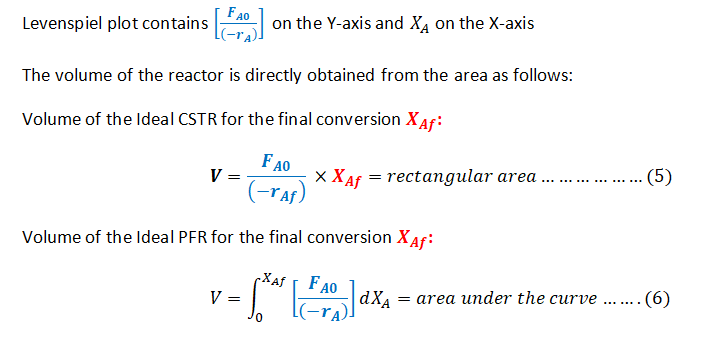

Step by step
Solved in 4 steps with 8 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
based off this graph what would the volume of the PFR be if the PFR conversion is 65%?
so CSTR conversion is still 20% but then followed by a PFR that is 65%
Solution
Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The