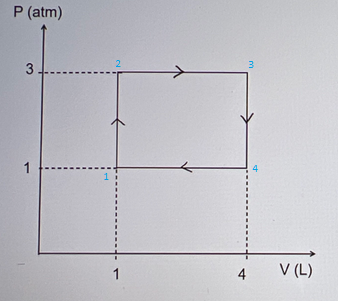
An ideal gas is taken through a cycle as shown in the figure. What is the net work done on the gas per cycle? P (atm) 3 1 O-608 J O -6.0 J Not enough information 608 J 1 O 6.0 J 4 V (L)
Q: Three ideal gas systems are prepared, each containing 1.0 mole of ideal gas at pressure 1 atm and…
A:
Q: In the shown Figure, the net work done by the gas during the close cycle is equal to: p (P.) 6x10…
A:
Q: The heat engine shown in the figure uses 2.0 mol of a monatomic gas as the working substance.…
A:
Q: Multiple thermodynamic paths are shown above. Assume an ideal gas in a piston/chamber scenario. For…
A: The piston would move outward when there is increased in volume.
Q: A gas in a cylinder undergoes change from an initial state i to a final state f shown in the figure…
A:
Q: Which figure represents the path for the work on the gas, if the work was given by: W=P,(V-V)?
A:
Q: 50.00 moles of a monatomic ideal gas increases in volume as shown below. Its final pressure is 4.50…
A:
Q: A gas undergoes a change of state described by the pV diagram shown in the figure below. 4.00- pi10²…
A: Work done is area under PV diagram. from the figure area=12(3-1)×102×(3-1)+2×102×1 =400 J…
Q: (a) Find the work done by an ideal gas as it expands from point A to point B along the path shown in…
A: The area under the graph in a PV diagram is equal to the work done on the gas. If the final volume…
Q: Multiple thermodynamic paths are shown above. Assume an ideal gas in a piston/chamber scenario. For…
A:
Q: 5 moles neon gas is expanded isothermally. Molar mass of neon gas is approximately 20g. Gas constant…
A: Since neon is a monatomic gas, we can find its internal energy using:Internal Energy Where:n =…
Q: An ideal Carnot heat engine operates between 385 K and 460 K. What is its efficiency? O 0.61 O 0.38…
A: Temperature of sink ( T1 ) = 385 KTemperature of heat reservior( T2 ) = 460 K
Q: A gas expands from V0 to V1 at constant pressure p0. How much work W is done by the gas?
A: The objective of the question is to calculate the work done by a gas when it expands from an initial…
Q: A gas in a cylinder undergoes change from an initial state i to a final state f shown in the figure…
A: The work done by gas is calculated by using the formula W=P∆V , where P is the pressure and V is the…
Q: p(atm) 1+ tv(L) 1 What is the work done by the gas when it undergoes the isobaric (constant…
A: Given data: Constant pressure (P) = 2 atm Initial volume (Vi) = 1 L Final volume (Vf) = 5 L…
Q: n ideal gas with energy E = NKBT is subjected to a cyclic, quasi-static (i he figure. What is the…
A: An ideal gas with energy E = 52NkBT is subjected to a cyclic, quasi-static (i.e. reversible) process…
Q: When a gas expands adiabatically, the temperature of the gas remains constant. O the internal…
A: please see the next step for solution
Q: A gas is heated and allowed to expand such that it follows a horizontal line path on a pV diagram…
A:
Q: an ideal gas. The pressure is doubled as seen on th p (kPa) 30.0 V (m³) 0.050
A:
Q: One pound of air per second with an initial temperature of 180°F is allowed to expand without flow…
A:
Q: e volume changes between V1 and 3V1 as shown. e total work done by the gas during the process from…
A: The area under the P-V diagram gives the work done by the gas. W=PVWhere,P=PressureV=Volume
Q: 3 moles of O2 at 1 atm and 500K expands isothermally to 0.3 m3. What is the change in entropy of the…
A: ...
Q: Helium (He), a monatomic gas, fills a 0.039 m³ container. The pressure of the gas is 5.0 x 105 Pa.…
A:

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- the volume of an ideal gas is decreased from 5L to 5mL at a constant pressure of 1 atm. Calculate the work associated with this process.function of volume by n(V)=CV2, where C is a constant. What is the correct expression for the work done on the gas for this process? (hint: put the expression for the molesA gas expands from 2.2 L to 3.6 L against a constant external pressure of 1.6 atm. What is the work done? A. -227 J B. 227 J C. 2.24 J D. -2.24 J
- The heat engine shown in the figure uses 2.0 mol of a monatomic gas as the working substance. (Figure 1) igure p (kPa) 600- 400 200 0 0 0.025 0.050 V (m³) 1 of 1 Part E part. What is the engine's thermal efficiency? Express your answer using two significant figures. η = Submit VE ΑΣΦ Request Answer ? %When the volume of an ideal gas stays the same, which of the followings must be true? Select all apply.Consider the process shown in (Figure 1). How much work is done on the gas in this process? p (kPa) 400- 200- i 0 0 100 200 300 V (cm³)
- One mole of an ideal gas, initially at 310 K, is cooled at constant volume so that the final pressure is one-sixth of the initial pressure. Then the gas expands at constant pressure until it reaches the initial temperature. Determine the work done on the gas. -1.93 X kJ Additional Materials eBookAs shown below, a nonideal gas goes through the cycle ABCA. During the process AB, 71.5 J of heat was added to the gas. During the process BC, 8.2 J of heat was removed from the gas. Determine WABCA & QCA. WABCA = QCA P(N/m²) 10 2 2 A 4 6 8 B с 10 V (m³)tab Consider the following figure. (The x axis is marked in increments of 2.5 m³.) P (Pa). esc caps lock 6 x 106 4 X 106 2 x 106 V (m³) 1 (a) Determine the work done on a gas that expands from i to f as indicated in the figure. MJ (b) How much work is performed on the gas if it is compressed from f to / along the same path? MJ ! 1 F1 A NO 2 N FF 200 F2 W S # 3 80 F3 X E * D $ 4 F4 R C % 5 F MacBook Air T V の‥ 6 F6 G & 7 F7 H B 2 E