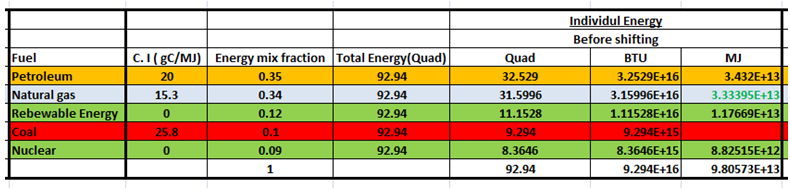
According to the U.S. Energy Information Agency, the total annual energy used in this country is 92.94 Quad (1 Quad = 1015 Btu) and our current energy mix is 35% petroleum, 34% natural gas, 12% renewable energy, 10% coal, and 9% nuclear power. The carbon intensity of each fuel is listed below. If all of the coal production was shifted to natural gas, what would the average carbon intensity be? gC/MJ 20 petroleum natural gas renewable 15.3 energy coal 25.8 nuclear
According to the U.S. Energy Information Agency, the total annual energy used in this country is 92.94 Quad (1 Quad = 1015 Btu) and our current energy mix is 35% petroleum, 34% natural gas, 12% renewable energy, 10% coal, and 9% nuclear power. The carbon intensity of each fuel is listed below. If all of the coal production was shifted to natural gas, what would the average carbon intensity be? gC/MJ 20 petroleum natural gas renewable 15.3 energy coal 25.8 nuclear
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
If he process was also shown not just answer

Transcribed Image Text:According to the U.S. Energy Information Agency, the total annual energy used in this country is 92.94 Quad (1 Quad = 10 15
petroleum, 34% natural gas, 12% renewable energy, 10% coal, and 9% nuclear power. The carbon intensity of each fuel is listed below. If all of the coal production was
shifted to natural gas, what would the average carbon intensity be?
Btu) and our current energy mix is 35%
gC/MJ
petroleum
natural gas
20
15.3
renewable
energy
coal
25.8
nuclear
Expert Solution
Contribution from Individual Energy sources to the Total energy(Before shifting)
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The