a. Lewis structure b. Drawing of shape (label angles) c. Name of shape d. Polar or nonpolar? e. Hybridization NH, a. Lewis structure b. Drawing of shape (label angles) c. Name of shape d. Polar or nonpolar? e. Hybridization

CH4- It is called methane. It is made up of 1 atom of carbon and 4 atoms of hydrogen. It is group 14 hydride and the alkane and is the main constituent of natural gas.
(A) Lewis Structure - It is the diagram which shows bonding between atoms and lone pairs of the electron within the molecule.
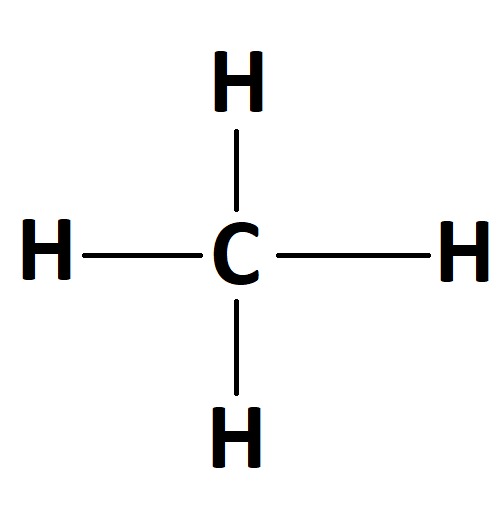
(B) Drawing of shape
The shape of the CH4 molecule is tetrahedral because it has 4 pairs of lone pairs. The Bond angle between H-C-H is 109.5 degrees.
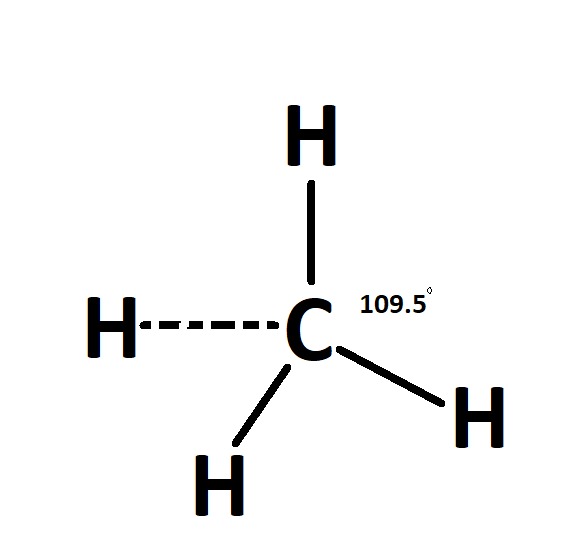
(C) The name of the shape is tetrahedral because it has 4 pairs of lone pairs.
(D) All the outer atoms are the same, and the dipole moments are in the same direction that is in the direction of carbon atom therefore overall molecule becomes non-polar. CH4 is Non-Polar.
(E) Hybridisation of CH4 molecule is sp3.
Step by step
Solved in 2 steps with 4 images









