A student is testing a different concentration of Cu(SO4)2, but finds that his absorbance readings are too high for the instrument. What steps can he take to measure the absorbance accurately?
A student is testing a different concentration of Cu(SO4)2, but finds that his absorbance readings are too high for the instrument. What steps can he take to measure the absorbance accurately?
Beer’s lambert law states that the amount of light absorbed is directly proportional to the concentration of the substance and path length of light through the solution. The expression for beer’s lambert law in which A represents the absorbance, Epsilon represents molar extinction coefficient, l represents length of the sample tube and c is the concentration of the solution is represented as follows:
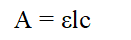
According to the Beer’s Lambert Law, the absorbance is directly proportional to the concentration of the solution that means if the solution is highly concentrated then the absorbance value will come high. Thus, to obtain a low value or accurate value of absorbance the solution must be diluted.
Step by step
Solved in 3 steps with 1 images









