(a) Plot C(t) as a function of time. (b) Plot E(t) as a function of time. (c) Calculate the mean residence time and variance.
(a) Plot C(t) as a function of time. (b) Plot E(t) as a function of time. (c) Calculate the mean residence time and variance.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question

Transcribed Image Text:Q5.
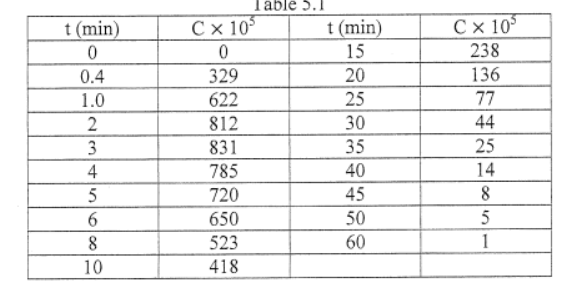
A pulse test gave the concentration measurements as shown in Table 5.1 at the outlet:
Table 5.1
t (min)
C x 10
t (min)
C x 10°
15
238
0.4
329
20
136
1.0
622
25
77
2
812
30
44
3
831
35
25
4
785
40
14
5
720
45
8
6
650
50
5
8
523
60
10
418
(a)
Plot C(t) as a function of time.
(b)
Plot E(t) as a function of time.
(c)
Calculate the mean residence time and variance.
Expert Solution
Step 1
this is the table given in the question where we had to find the E(t) at the very first moment which is equal to
here the value of .
| t(min) | E(t) |
| 0 | 0 |
| 0.4 | 0.052741 |
| 1 | 0.099711 |
| 2 | 0.13017 |
| 3 | 0.133216 |
| 4 | 0.125842 |
| 5 | 0.115422 |
| 6 | 0.1042 |
| 8 | 0.083841 |
| 10 | 0.067009 |
| 15 | 0.038153 |
| 20 | 0.021802 |
| 25 | 0.012344 |
| 30 | 0.007054 |
| 35 | 0.004008 |
| 40 | 0.002244 |
| 45 | 0.001282 |
| 50 | 0.000802 |
| 60 | 0.00016 |
this the distribution of E(t) vs time (min)
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The