A hypothetical atom has atomic number 71. Using the Bohr hypothesis, find the frequency of revolution for the electron in the 18th energy level, in Hz.
Question in images

According to the Bohr hypothesis
1
The centripetal force on the electron in an orbit is equal to the Coulomb force between nucleus and electron. Thus
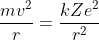
Where m = mass of the electron, e = magnitude of the charge on the electron, Z = atomic number of the atom,
r = distance between electron and nucleus, v = Speed of the electron in an orbit, k = Coulomb's constant
2
The orbit angular momentum of the revolving electron in an orbit is quantized. Thus
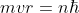
Where n = 1, 2, 3 ........ and 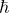 = Reduced planck constant
= Reduced planck constant
The atomic number of an atom is Z = 71
Using Bohr hypothesis
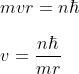 ............................(1)
............................(1)
Now
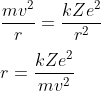
Using equation (1)
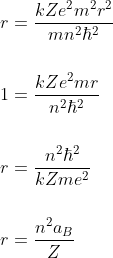
Where
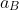 = Bohr radius = 0.529 × 10- 10 m
= Bohr radius = 0.529 × 10- 10 m
Using equation (1)
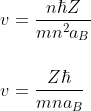
The frequency of revolution of the electron is given by
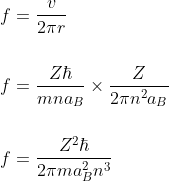
For 18th energy level n = 18
m = 9.11 × 10- 31 kg
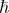 = 1.05457 × 10- 34 J.s
= 1.05457 × 10- 34 J.s
Thus
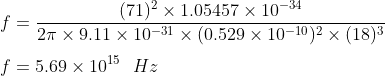
Step by step
Solved in 3 steps with 11 images
