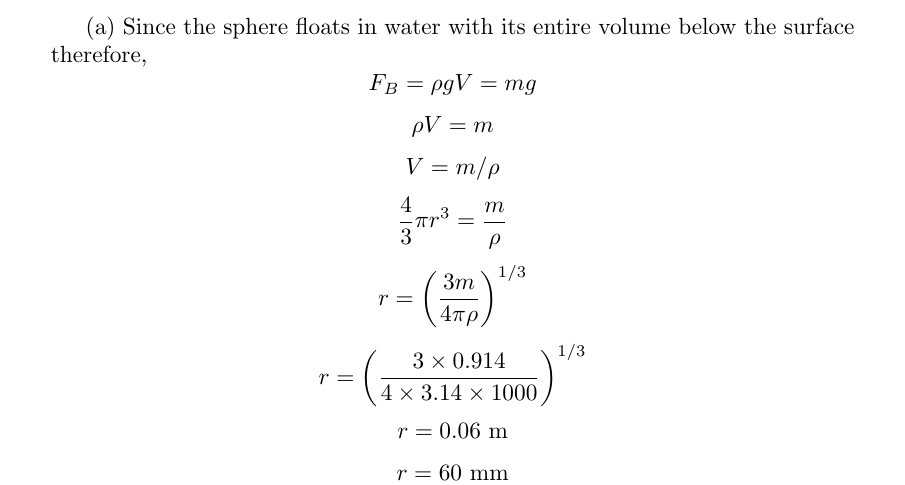
A hollow silver (?Ag = 1.050 ✕ 104 kg/m3) spherical shell of mass m = 0.9140 kg floats in water with its entire volume below the surface. (Assume the temperature of the water is 4°C. Enter your answers to at least four significant figures.) (a) What is the radius of the sphere? mm (b) What is the thickness of the shell wall? mm
Q: Large helium-filled balloons are used to lift scientific equipment to high altitudes. (a) What is…
A: Given T1=10.8° C + 273.15 = 283.95 K T2 = -43.7° C + 273.15 = 316.85 K Ideal gas law equation…
Q: 1. Large helium-filled balloons are used to lift scientific equipment to high altitudes. (a) What is…
A:
Q: A balloon has a volume of 2.0 m3. As it rises in the Earth's atmosphere, its volume expands. What…
A: Initial volume, V1 = 2 m3Initial pressure, P1 = 1 atmInitial temperature, T1 = 20°C = 293 KFinal…
Q: An arctic weather balloon is filled with 40.7 L of helium gas inside a prep shed. The temperature…
A: The Charles law is given as, V1T1=V2T2V2=V1T2T1
Q: A bigh-altitude balloon is partially filled with 3 m of helium at sea level. As the balloon ascends…
A: Boyle's law define that the product of pressure and volume remains constant. P1V1 = P2V2 P1 = 1 atm…
Q: A copper flask is filled to the brim with mercury. The flask and the mercury have an initial…
A: Given: Initial temperature of both flask & mercury, t=15.7 °C Temperature given to both flask…
Q: A tube has a length of 0.043 m and a cross-sectional area of 6.5 x 10-4 m2. The tube is filled with…
A: Given data: Length of tube, L=0.043 m Cross-sectional area, A=6.5×10-4m2 Diffusion constant of…
Q: B lc A la Wa Given: The foam's density: p = 130 kg/ m³ The foam's thickness: t = 0.6 c m . Assume…
A: Let ρ denotes the density, lA denotes the plate A’s length, wA denotes the plate A’s width, t…
Q: At 37 m below the surface of the sea (density of 1422 kg/m3 ), where the temperature is 6◦C, a diver…
A:
Q: Using the same sample of gas (P = 325 torr ,T = 14 °C), we wish to change the pressure to 3250 torr…
A: As per guidelines we are suppose to do only one question from multiple questions, please post other…
Q: At standard temperature and pressure, a gas has a density of 0.088 kg/m3. What volume (in m3) does…
A: Here, We have to find the volume ( in m3 ) that 2.55 kg of hydrogen will occupy at standard…
Q: A bicycle tire has a pressure of 6.75 × 105 Pa at a temperature of 19°C and contains 2.00 L of gas.…
A: Solution:-Concept and formulas used:-1) According to Ideal gas equation, we havePV = nRTHere, P =…
Q: diver is at 5 meter depth in a fresh water lake. At that depth, the temperature is 15◦C. The…
A: Hi please find solution to your problem
Q: The volume of an automobile tire is 2.5x10^-2m3. The pressure of the air in the tire is 3.3 atm and…
A: Given data *The volume of an automobile tire is V = 2.5 × 10-2 m3 *The pressure of the air in the…
Q: Large helium-filled balloons are used to lift scientific equipment to high altitudes. (a) What is…
A: Given that, Initial temperature (T1)= 22.8°C =( 273.15 + 22.8 ) K T1= 295.95 K Final temperature…
Q: A basketball is pressurized to a gauge pressure of PG = 55 kPa when at the surface of a swimming…
A: Given that: PG=55 kPaPatm=101 kPa ρ = 1000 kg/m3
Q: A vehicle tire is inflated with air initially at 15°C and normal atmospheric pressure. During the…
A: a) Given info : The initial pressure is Pi=1 atm. The initial and final temperaturesare Ti=15°C and…
Q: What will its pressure be, in pascals, if you let out an amount of air that has a volume of 105 cm3…
A: We know that the ideal gas equation as PV = nRTn= PVRTwhere P is pressure of the ideal gass V is the…
Q: A hot air balloon uses the principle of buoyancy to create lift. By making the air inside the…
A: Let m be the mass of the air inside the balloon.
Q: 1. Large helium-filled balloons are used to lift scientific equipment to high altitudes. (a) What is…
A:
Q: When air expands adiabatically (without gaining or losing heat), its pressure Pand volume V are…
A: Given Volume V = 540 cm3 = 540 m3 Pressure P = 91 kPa = 91 x 103 Pa Pressure changing rate dP/dt =…
Q: (b) What is the gauge pressure? (Enter your answer in atm and to at least 3 decimal places. Assume…
A: Formula:- Pabs= Pg + Patm Pg =Pabs - Patm ( Pg is gauge pressure, Pabs is absolute pressure,…
Q: You are working in a controlled environmental laboratory. For the experiments with which you are…
A:
Q: The mass of a hot-air ballon and its occupants is 300 kg (excluding the hot air inside the balloon).…
A: We are given pressure inside the balloon. We have to find the temperature of the heated air inside…
Q: A gas is in a sealed container. The gas pressure is tripled and the temperature is doubled. By what…
A:
Q: hollow silver (?Ag = 1.050 ✕ 104 kg/m3) spherical shell of mass m = 0.9840 kg floats in water with…
A:
Q: A cylindrical tank with a diameter of 1.0 m and a length of 3.0 m holds oxygen gas (O2) at a…
A:
Q: A water molecule has a diameter of about 0.275 nm. How many hours would it take for a water molecule…
A:
Q: When air expands adiabatically (without gaining or losing heat), its pressure P and volume V are…
A:
Q: Large helium-filled balloons are used to lift scientific equipment to high altitudes. (a) What is…
A:
Q: Rectangular tank contains 3.0 kg of air. The length of the tank is 1.1 m and the width is 0.9 m. The…
A:
Q: what is is the mass of air in grams
A: Given data: The volume of automobile tire is: V = 2.5×10-2 m3 The pressure of air in the tire is: P…
Q: A volume of 58.0 L of hydrogen is heated from 33°C to 68°C. If its original density is 4.85 kg/m3…
A: Given that,The initial volume, V1=58×10-3Initial temperature, T1=33°CThe final temperature,…
Q: A hot air balloon uses the principle of buoyancy to create lift. By making the air inside the…
A:
Q: Large helium-filled balloons are used to lift scientific equipment to high altitudes. (a) What is…
A:
Q: The volume of an automobile tire is 2.5 × 10−2m3 . the pressure of the air in the tire is 3 atm and…
A: According to ideal gas law - Where P = Pressure V = Volume n = Number of moles T = Temperature…
Q: When air expands adlabatically (without gaining or losing heat), Its pressure P and volume V are…
A: Differentiate the given equation with respect to time.…
A hollow silver
spherical shell of mass
floats in water with its entire volume below the surface. (Assume the temperature of the water is 4°C. Enter your answers to at least four significant figures.)
mm
(b) What is the thickness of the shell wall?
mm
Step by step
Solved in 2 steps with 2 images

- An ideal gas in a 1.25-gallon[gal] container is at a temperature of 135 degrees Celsius [°C] and pressure of 2.4 atmospheres [atm]. If the gas is oxygen (formula: O2, molecular weight=32 grams per mole [g/mol]), what is the amount of gas in the container in units of grams [g]?A basketball is pressurized to a gauge pressure of PG = 55 kPa when at the surface of a swimming pool. (Patm = 101 kPa). The ball is then submerged in the pool of water which has a density ρ = 1000 kg/m3. Assume the ball does not change in mass, temperature, or volume as it is submerged. Calculate the absolute pressure inside the basketball in kPa when it is at the surface. Write an equation for the pressure difference ΔP between the inside and outside of the ball when it is submerged a distance y below the surface of the water. Solve the pressure equation for the depth (in meters) at which the pressure difference between the inside and outside of the ball will become zero. At this depth the pressure inside the basketball is the same as the pressure outside the ball.Large helium-filled balloons are used to lift scientific equipment to high altitudes. What is the pressure inside such a balloon (in atm) if it starts out at sea level with a temperature of 20.8°C and rises to an altitude where its volume is sixteen times the original volume and its temperature is −35.7°C? (Enter your answer to at least 3 decimal places) What is the gauge pressure? (Enter your answer in atm and to at least 3 decimal places. Assume atmospheric pressure is constant.)
- At standard temperature and pressure, a gas has a density of 0.089 kg/m3. What volume (in m3) does 1.84 kg of hydrogen occupy at standard temperature and pressure [round your final answer to one decimal place]?Suppose you have a closed rigid container of air at 20 Celsius on the surface of the Earth. We increase the temperature of the container to 77, leaving it closed, and read a pressure gauge attached to the container. Initially the reading was 101 KPa (kilopascals, or 1000 N/m2). What is the new reading? Give your answer in KPa.A hot air balloon uses the principle of buoyancy to create lift. By making the air inside the balloon less dense then the surrounding air, the balloon is able to lift objects many times its own weight. A large hot air balloon has a maximum balloon volume of 2090 m3. a. If the air temperature in the balloon is 54 °C, how much additional mass, in kilograms, can the balloon lift? Assume the molar mass of air is 28.97 g/mol, the air density is 1.20 kg/m3, and the air pressure is 1 atm.