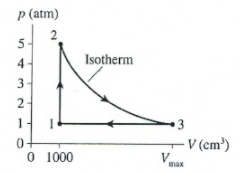
A heat engine using 0.03 moles of helium follows the cycle shown in the diagram. What is the volume Vmax in m3?
A heat engine using 0.03 moles of helium follows the cycle shown in the diagram. What is the volume Vmax in m3?
Related questions
Question
A heat engine using 0.03 moles of helium follows the cycle shown in the diagram. What is the volume Vmax in m3?

Transcribed Image Text:p (atm)
2
5-
4-
Isotherm
3-
2-
1-
1.
3
0+
0 1000
V (cm³)
V
max
Expert Solution
Step 1
Given:
A heat engine using 0.03 moles of helium.
The diagram is as follows:
Introduction:
The isothermal process, which is a thermodynamic process in which the temperature of a system remains constant. The transfer of heat into or out of the system happens so slowly that thermal equilibrium is maintained.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images
