
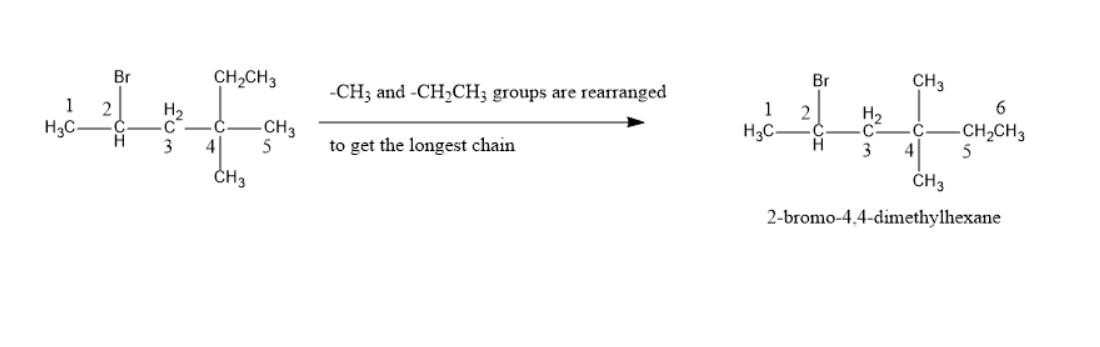
a) According to IUPAC rule, the nomenclature is done in such a way so that
i)the compound has the longest no of carbon chain. Here in longest chain total no of carbons is 6. So parent chain is Hexane.
ii) the numbering is started from the carbon which is closest to functional group Br. So the numbering in a) molecule is started from the left side carbon. So Br containing C-atom is C-2 and two Me-group containing carbon is C-4. It is a hexane chain.
iii) In the nomenclature the name of groups are arranged in alphabetical order. So, Br (bromo) comes first, then Me group(methyl) comes in the nomenclature.
iv) Prefixes like di, tri, tetra, penta etc. are used to indicate the groups of same type.
So the nomenclature is 2-bromo-4,4-dimethylhexane.
Step by step
Solved in 2 steps with 2 images









