A 7.98 g sample of an aqueous solution of nitric acid contains an unknown amount of the acid. If 20.8 mL of 2.43 M sodium hydroxide are required to neutralize the nitric acid, what is the percent by mass o nitric acid in the mixture? % by mass Submit Answer Retry Entire Group 9 more group attempts remaining
A 7.98 g sample of an aqueous solution of nitric acid contains an unknown amount of the acid. If 20.8 mL of 2.43 M sodium hydroxide are required to neutralize the nitric acid, what is the percent by mass o nitric acid in the mixture? % by mass Submit Answer Retry Entire Group 9 more group attempts remaining
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 100E: Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory as a...
Related questions
Question

Transcribed Image Text:**Problem Statement:**
A 7.98 g sample of an aqueous solution of nitric acid contains an unknown amount of the acid. If 20.8 mL of 2.43 M sodium hydroxide are required to neutralize the nitric acid, what is the percent by mass of nitric acid in the mixture?
**Input Box:**
- % by mass (text field for input)
**Buttons:**
- Submit Answer
- Retry Entire Group
**Additional Information:**
- 9 more group attempts remaining
**Notes:**
- Use the References to access important values if needed for this question.
This problem involves a stoichiometry calculation requiring you to determine the percent by mass of nitric acid in a mixture using the concept of neutralization with sodium hydroxide.
Expert Solution
Step 1
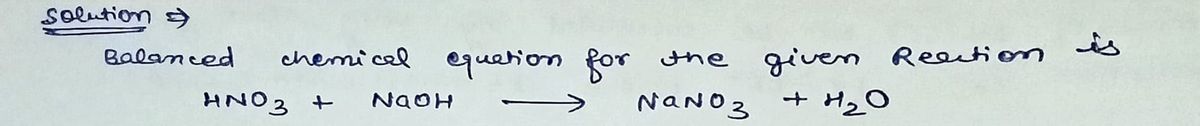
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning
