A 5.186 gram sample of the anti-malarial pesticide DDT was dissolved in 100.0 mL of alchohol and 10.00 mL aliquots were taken for analysis. One such aliquot was decomposed with metallic sodium, and the liberated chloride ion was precipitated as AgCl. Express the results of this analysis in terms of %DDT (C14H9Cl5, FW = 354.49) based on the recovery of 150 mg of AgCl (FW = 143.32).
A 5.186 gram sample of the anti-malarial pesticide DDT was dissolved in 100.0 mL of alchohol and 10.00 mL aliquots were taken for analysis. One such aliquot was decomposed with metallic sodium, and the liberated chloride ion was precipitated as AgCl. Express the results of this analysis in terms of %DDT (C14H9Cl5, FW = 354.49) based on the recovery of 150 mg of AgCl (FW = 143.32).
The structure of DDT is as shown below:
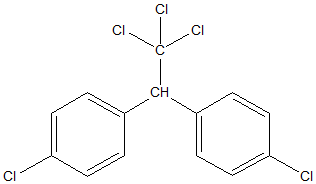
One molecule of DDT has 5 atoms of Cl.
Fusion of DDT with sodium will liberate its Cl atoms as chloride ions.
One molecule of DDT on reaction with metallic sodium will form five chloride ions. These ions will react with silver nitrate will form AgCl as precipitates.
So, a molecule of DDT will form 5 molecules of AgCl.
As molar mass of DDT is 354.49 g/mol and that of AgCl is 143.32 g/mol.
So, 354.49 g of DDT will precipitate 5*143.32 g = 716.60 g of AgCl.
Mass of AgCl recovered from sample is 150 mg, calculate the mass of DDT in the sample as shown below:
So, the actual amount of DDT in sample is 0.07420 g.
Step by step
Solved in 3 steps with 1 images









