7. Freezing point of pure Naphthalene from cooling curve- 8. Freezing point of solution 9. AT 10. Molality of solution, m I1. Molar mass of Unknown 12. Include graphs of cooling curves CALCULATIONS
7. Freezing point of pure Naphthalene from cooling curve- 8. Freezing point of solution 9. AT 10. Molality of solution, m I1. Molar mass of Unknown 12. Include graphs of cooling curves CALCULATIONS
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 7P
Related questions
Question

Transcribed Image Text:Data Sheet
UNKNOWN NUMBER:
1
49.83
4575
8.08
12.47
I1.031
1.44
1. Mass of test tube + naphthalene
2. Mass of empty test tube
%3D
3. Mass of naphthalene
4. Mass of vial + Unknown
%3D
5. Mass of vial
%3D
6. Mass of Unknown
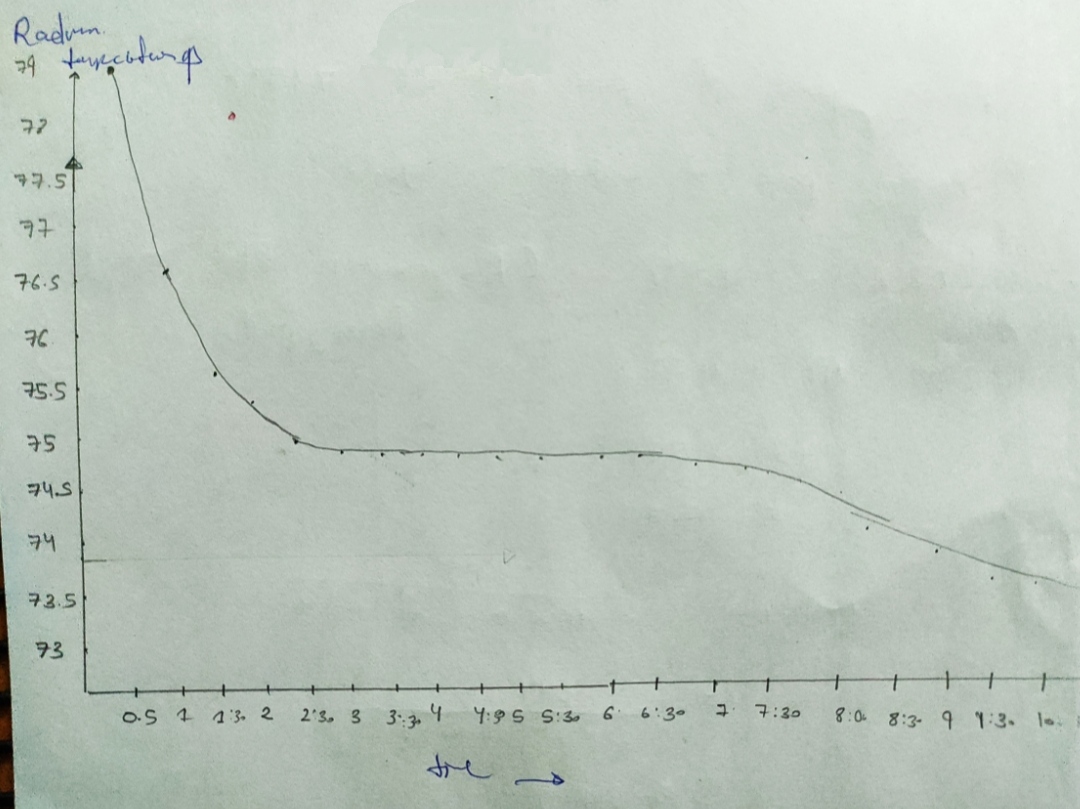
Cooling curve data
Pure Naphthalene
Naphthalene + Unknown
Temp
Time
Temp
Time
Temp
Time
Temp
Time
79 0.5
T4-9 5
20:7 5:30
78:2 020
749 S:30
85.2 30
84.5
B15 1:30
70.9 6
6.70
72 30
14.9 6
14.9 6:30
75.6 1:08C 74 9
75:52
20.0
69.8 7.
69.4 7:30
68.9
68:3
76:5. Imit
74
75.02.9
749,3.
28.2 2
76 Y
25.0
22. 3.
.
2:10
"NS 130
3:30 24.0 9
23-4 9:30
74.9.4.30 23.5
8:)0
679 9
67.2 9.30
.
21.8
71.4 4
330
74.9
94.9 u
१:१०
:30 67.2 10
20.9 S
b6.5 10.30
75-5
750
10:50
66.0 1
7. Freezing point of pure Naphthalene from cooling curve =
8. Freezing point of solution
9. AT
10. Molality of solution, m
11. Molar mass of Unknown
12. Include graphs of cooling curves
CALCULATIONS
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
Recommended textbooks for you



