4- Barium titanate BaTiO3 has a phase with structure as in the attached Figure, which the Ba (Ba; 137 g/mol) atoms are at corners of a cube with a Ti (Ti; 48 g/mol) atom at the center and Oxygen (O; 16 g/mol) atoms at the center of each face of the cube. The mass density of BaTiO3 p= 6.02 g/cm³. If BaTiO3 powder is exposed to X-rays with = 0.154 nm in a Debye-Scherer experiment. (NA= 6.02x1023 mol-¹). a- Calculate the number of atoms per unit cell? b- What are the coordinates of the atoms Br-Ti-O? c- Calculate the lattice constant (a)? Ba O Ti
4- Barium titanate BaTiO3 has a phase with structure as in the attached Figure, which the Ba (Ba; 137 g/mol) atoms are at corners of a cube with a Ti (Ti; 48 g/mol) atom at the center and Oxygen (O; 16 g/mol) atoms at the center of each face of the cube. The mass density of BaTiO3 p= 6.02 g/cm³. If BaTiO3 powder is exposed to X-rays with = 0.154 nm in a Debye-Scherer experiment. (NA= 6.02x1023 mol-¹). a- Calculate the number of atoms per unit cell? b- What are the coordinates of the atoms Br-Ti-O? c- Calculate the lattice constant (a)? Ba O Ti
Related questions
Question

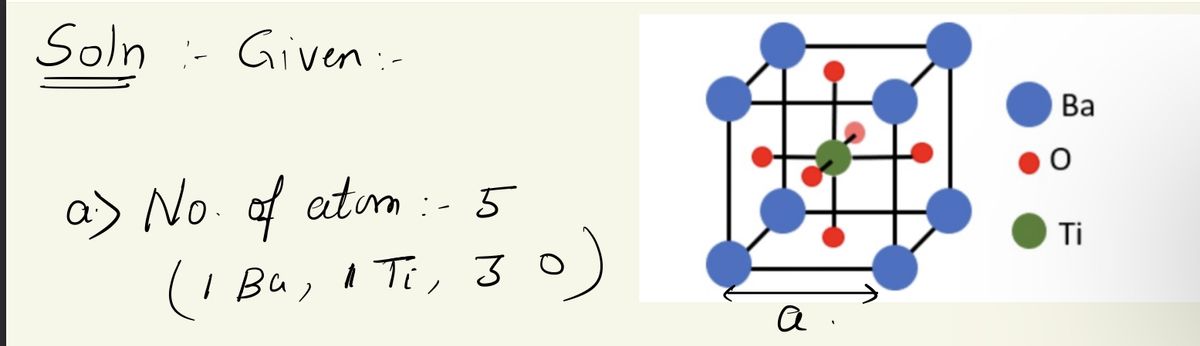
Transcribed Image Text:4- Barium titanate BaTiO3 has a phase with structure as in the attached Figure,
which the Ba (Ba; 137 g/mol) atoms are at corners of a cube with a Ti (Ti; 48
g/mol) atom at the center and Oxygen (O; 16 g/mol) atoms at the center of
each face of the cube. The mass density of BaTiO3 p= 6.02 g/cm³. If BaTiO3
powder is exposed to X-rays with = 0.154 nm in a Debye-Scherer
experiment. (NA= 6.02x10²³ mol-¹).
#
a- Calculate the number of atoms per unit cell?
b- What are the coordinates of the atoms Br-Ti-O?
Ba
O
i)
ii)
iii)
Ti
c- Calculate the lattice constant (a)?
d- Calculate the Bragg deflection angle for the first peak of:
The planes containing Ba and O only? (answer: 0= 11.09⁰)
The planes containing Ba, Ti and O? (answer: 0= 15.797°)
The planes containing Ti and O only? (answer: 0= 22.644°)
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images
