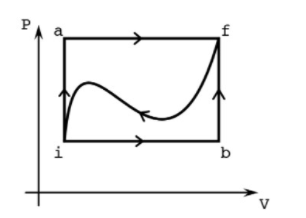
3 The First Law P a f i b V When a system is taken from state i to state f along the path iaf, the heat added to the system is Q = 50 cal and the work done by the system is W = 20 cal. Along the path ibf, Q = 36 cal. (a) What is W along the path ib f? (b) If W = -13 cal for the curved return path fi, what is Q for this path? (c) If E = 10 cal, what is E,? (d) If E, = 22 cal, what is Q for the process ib? %3D (e) If E, = 22 cal, what is Q for the process bf? %3D
3 The First Law P a f i b V When a system is taken from state i to state f along the path iaf, the heat added to the system is Q = 50 cal and the work done by the system is W = 20 cal. Along the path ibf, Q = 36 cal. (a) What is W along the path ib f? (b) If W = -13 cal for the curved return path fi, what is Q for this path? (c) If E = 10 cal, what is E,? (d) If E, = 22 cal, what is Q for the process ib? %3D (e) If E, = 22 cal, what is Q for the process bf? %3D
Related questions
Question

Transcribed Image Text:3 The First Law
a
f
i
b
V
When a system is taken from state i to state f along the path iaf, the heat added to the system is Q = 50 cal and the work
done by the system is W = 20 cal. Along the path ibf, Q = 36 cal.
(a) What is W along the path ib f?
(b) If W = -13 cal for the curved return path fi, what is Q for this path?
(c) If E = 10 cal, what is E,?
(d) If E, = 22 cal, what is Q for the process ib?
%3D
(e) If E, = 22 cal, what is Q for the process bf?
Expert Solution
Step 1
Step by step
Solved in 2 steps with 1 images
