21) Vanadium has two naturally occurring isotopes, 50V with an atomic mass of 49.9472 amu and 51v with an atomic mass of 50.9440. The atomic weight of vanadium is 50.9415. The percent abundances of the vanadium isotopes are % 51v % 50V and
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
I'm confused on how to get the
It's not as straight forward as adding the two given and dividing by two is it?
The question is asking for the % abundance if that isn't clear.

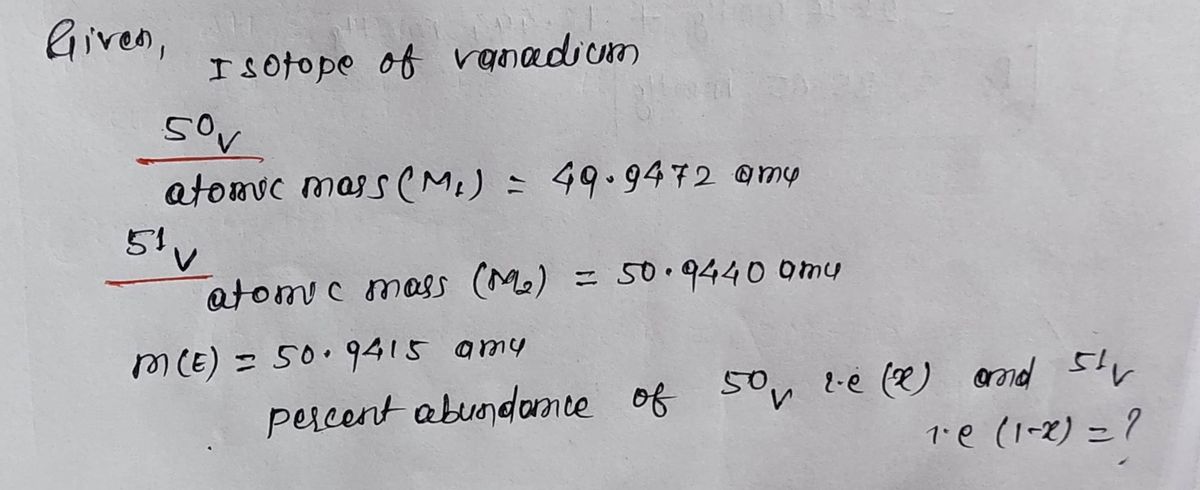
Step by step
Solved in 2 steps with 2 images

Why does the x go from:
-0.9968x = -0.0025
To:
x= 0.0025x/0.9968
And not:
x= 0.9968/0.0025
Wouldn't the negatives simply be ignored and the x would remain with:
0.9968x and then you divide then 0.0025 without the coefficient x to get the value of x by cancelling out the 0.9968?








