Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
The answer is E.) 5.79 x 10^22.
I don't know if my lack of rounding appropriate numbers is giving me incorrect answers but I'm sure I've done everything correctly.

Transcribed Image Text:13) How many oxygen atoms are contained in 2.74 g of aluminum sulfate?
A) 12
B) 6.02 x 1023
C) 8.01 x 10-3
D) 7.22 x 1024
E) 5.79 x 1022
13)

Transcribed Image Text:look @ 23
Unit 3 Hin (cont.) (Do I need to round?
and?)
-2
13.) how many 0 atoms are contained in 2.74g of aluminum sulfate. A1² 504 ²
A1: 26.9813386 (2) 53.9638772
22
E.) s.79x10²
5:32.065
10:13.9994
wwwwwwwww
12.74g. 1 mol: Al. 504
(1) = 32.655 g/mal
(4) =
63.9776gland)
* PROBLEMY (It's not 4.63 it's 4-15.49)
15.9994
63-99269_11688227-463
%
g derve
15.0.8256772g/mol
MAHARVARAPAREMATANDSVIN
(Okay hold on... AGAIN I don't understand.)
WIVAAAAM
www.w
hmm
C
100
2.745 15³1 A1₂ 504
=
convert to male, convert to mole, convert to mole convert to mole
7.
16882246
274
mand
4 moli
1 mol: A1² 504
150.0256772
que
to get close to the answer I can subtract 6.022 but
il must be setting it up wining.
43810
158.8256772.glimal 1 mal Al 504
4 mol: 0
do you... 2.74-1.168822746? I don't think 30
42.65776445%
.
why? I am
0-292205687
15.99949
1m610
n
6.2922 85687
2.74
↓
11.7998832% 0
50...
so lost.
175.353424
158.8256772
=
(180)
1.1168822746
Expert Solution
Step 1
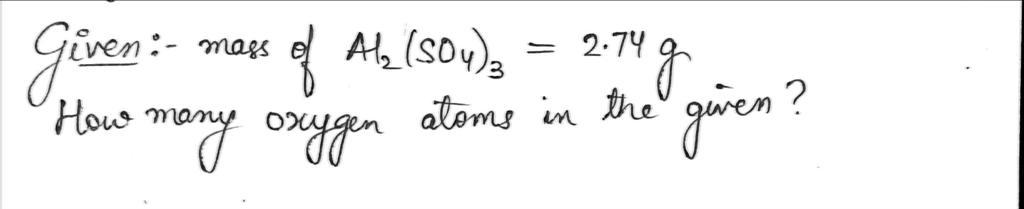
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY