1. What is the PH of a 0.1 molar solution of acetic acid ? To what volume must 1 liter of this solution be diluted so that the PH of the resulting solution will be twice the original value? Given (K, = 1.8x10-5). %3D CH,COOH + CH,COO +H*
Hello, i need help with this qustion please.

The dissociation reaction taking place is
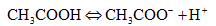
Given : initial concentration of CH3COOH = 0.1 M
Assuming y concentration of CH3COOH dissociates as per the reaction.
Hence the concentration of ions formed at equilibrium are
[H+ ] = y = [CH3COO- ]
And concentration of CH3COOH remaining at equilibrium = initial - dissociated = 0.1 - y
Since the Ka of CH3COOH <<< 1
Hence we can assume negligible amount of CH3COOH will dissociate.
Hence the concentration of CH3COOH remaining at equilibrium = [CH3COOH ] = 0.1 - y = 0.1 M approx.
The dissociation constant expression for the reaction can be written as
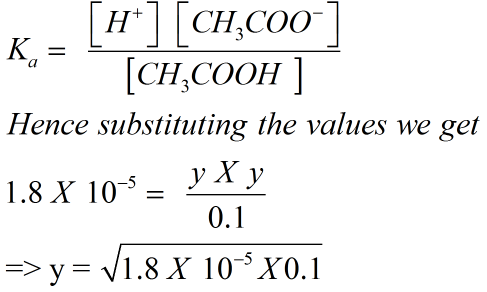
=> y = 1.34 X 10-3 M = [H+ ]
=> pH = -log[H+ ] = -log(1.34 X 10-3 ) = 2.875 approx.
Step by step
Solved in 5 steps with 3 images









