Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Kindly help me here. I have excluded some steps in my mechanism as I believe they are unnecessary. Help me decipher the major product and minor product and why.

Transcribed Image Text:O
1. Hg(OAc)2, H₂O/THF
2. NaBH4, ethanol
Methyl (E)-2-methylbut-2-enoate

Transcribed Image Text:Hg
OR
H₂₂0
-H30* ķ
OH
OH
0
OR
reduction
by
Na BH u
+ enantiomer
major product
tv
H₂0
+ enantiomer
T
Ho
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Thank you for the answer. Kindly have a look at this textbook solution and explain wether it could be wrong or its something else.
Transcribed Image Text:Organic Chemistry Principles and Mechanisms 2e By Joel Karty - Copy.pdf - Adobe Acrobat Reader DC (32-bit)
File Edit View Sign Window Help
Home Tools
Cambrid...
D
At least one signature is invalid.
Bookmarks
凤
11.10 Kinetic versus
Thermodynamic
Control in
Electrophilic Addition
to a Conjugated
11.11 - Terpene
Biosynthesis:
Carbocation
Chemistry in Nature
12 - Electrophilic
Addition to Nonpolar
Bonds 2: Reactions
Involving Cyclic
Transition States
X
12.1 - Electrophilic
Addition via a
Three-Membered
Ring: The General
Mechanism
12.2 - Electrophilic
Addition of Carbenes:
Formation of
Solomon...
Organic ... x
617
Study_Gu...
(668 of 1587)
H₂O
HO
(a)
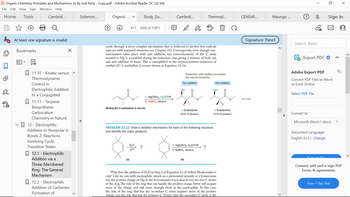
Methyl (E)-2-methylbut-2-enoate
ceeds through a more complex mechanism that is believed to involve free radicals
(species with unpaired electrons; see Chapter 25). Consequently, even though oxy-
mercuration takes place with anti addition, any stereochemistry of the C atom
bonded to Hg is scrambled during the reduction step, giving a mixture of both syn
and anti addition of water. This is exemplified in the oxymercuration-reduction of
methyl (E)-2-methylbut-2-enoate shown in Equation 12-24.
1. Hg(OAc)2, H₂O/THF
2. NaBH4, ethanol
?
Cambrid...
OH
Ou
Reduction with NaBH4 scrambles
the stereochemistry.
Syn addition
O
PROBLEM 12.12 Draw a detailed mechanism for each of the following reactions
and identify the major products.
H
+ Enantiomer
43% of product
(b)
Thermod...
1. Hg(OAc)2, H₂O/THF
2. NaBH4, ethanol
?
Anti addition
OH
O
+ Enantiomer
57% of product
Why does the addition of H₂O in Step 2 of Equation 12-23 follow Markovnikov's
rule? Like we saw with nucleophilic attack on a protonated epoxide or a bromonium
ion, the positive charge on Hg in the mercurinium ion is shared over the two C atoms
of the ring. The side of the ring that can handle the positive charge better will acquire
more of the charge and will more strongly draw in the nucleophile. In this case,
the side of the ring that has the secondary C atom acquires more of the positive
charge, not the side that has the primary C. Notice that the secondary C atom is the
CENG40...
Maungo...
Signature Panel
(12-24)
?
Search 'Bates'
T
Export PDF
Select PDF File
Adobe Export PDF
Convert PDF Files to Word
Excel Online
Convert to
Sign In
Microsoft Word (*.docx)
Document Language:
English (U.S.) Change
Colect Fil
Convert, edit and e-sign PDF
forms & agreements
Free 7-Day Trial
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY