1. a. A solution is created by mixing 35.3g of C&H12O6 with 100.0mL of water. (Molar Mass of CoH12Os = 180.18 g/mol). What is the molarity of the solution created assuming the volume of the water is the final volume of the solution as well?
1. a. A solution is created by mixing 35.3g of C&H12O6 with 100.0mL of water. (Molar Mass of CoH12Os = 180.18 g/mol). What is the molarity of the solution created assuming the volume of the water is the final volume of the solution as well?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
please show work for question #1

Transcribed Image Text:Section: Objective #11- Students can discuss the formation and properties solutions and can
solve problems involving solutions.
1. a. A solution is created by mixing 35.3g of CoH1206 with 100.0mL of water. (Molar Mass
of CSH1206 = 180.18 g/mol). What is the molarity of the solution created assuming the
volume of the water is the final volume of the solution as well?
b. Another solution is created by adding 75.0mL of water to 25.0mL of 1.50M NaCI. What is
the molarity of this new solution?
c. Which solution, the C6H12O6 (a) or the NaCl (b), will have the highest boiling point? Explain
your choice.
2. A cleaning solution calls for a 15% (v/v) mixture of cleaner in water. How much cleaner
and water are necessary to prepare 4.0 L of the solution?
EXPRESSING HOW MUCH IN
SOLUTION
Molarity (M)
mass of solute
mass of solution
mass %-
moles solute
Solutinn * 100%
M =
liters solution
volume . volume of solute
volume of solution
100%
When ciy amounts present
1 ppm = 1 mg/L
1 ppb = 1 pg/L
mass of solute
BO x 10
mass of solution
mass of solute
x 10
ppm naes of solutiom
ppb =
Exam 4 CHE 110 Fall 2020 10
3. Zinc and hydrochloric acid react according to the following balanced equation:
Zn(s) + 2 HCI(aq) → ZnCl2(aq) +H2(g).
What volume, in L, of 0.100 M hydrochloric acid will react with 35.5g of zinc?
A 1.5 M aq. NH4CI solution and a 1.25M aq. LizSO4 solution are separated by a
semipermeable membrane. Will the water flow toward the NH,Cl solution, or the
Li;SO, solution? How do you know?
4.
5. We have a saturated solution of CaCl2. Can we also say that it is a concentrated solution?
Explain your answer.
Expert Solution
Step 1
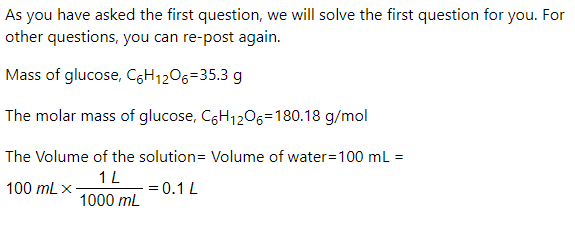
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY