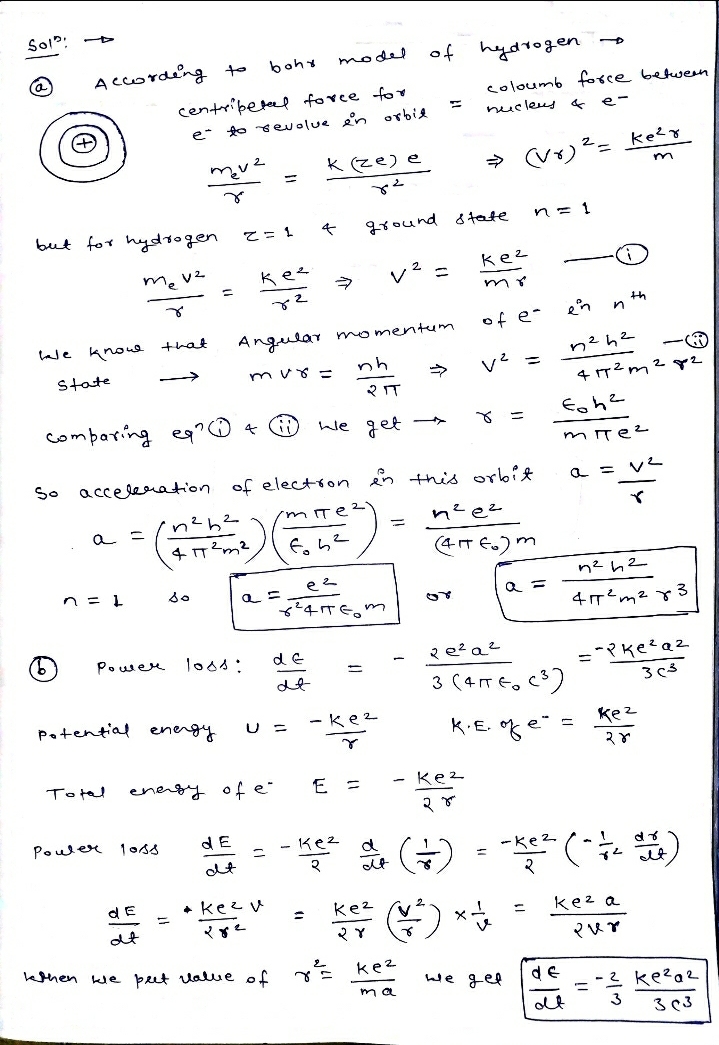
1) Consider the Bohr model of the hydrogen model (Z=1) in which an electron is in the smallest possible orbit (n=1). a) Find the acceleration of the electron in this orbit. b) Classical electromagnetic theory predicts that an electron with acceleration a will radiate energy. The power loss is given by = -2e²a²/(3(4¹c³)). Evaluate this formula for this atom where c is the speed of light in vacuum. c) If the electron continues to radiate at this rate how long will it take to reduce the energy from -13.6 eV to twice this value.
1) Consider the Bohr model of the hydrogen model (Z=1) in which an electron is in the smallest possible orbit (n=1). a) Find the acceleration of the electron in this orbit. b) Classical electromagnetic theory predicts that an electron with acceleration a will radiate energy. The power loss is given by = -2e²a²/(3(4¹c³)). Evaluate this formula for this atom where c is the speed of light in vacuum. c) If the electron continues to radiate at this rate how long will it take to reduce the energy from -13.6 eV to twice this value.
Related questions
Question

Transcribed Image Text:1) Consider the Bohr model of the hydrogen model (Z=1) in which an electron is in the
smallest possible orbit (n=1).
a) Find the acceleration of the electron in this orbit.
b) Classical electromagnetic theory predicts that an electron with acceleration a will
radiate energy. The power loss is given by = -2e²a²/(3(4¹c³)). Evaluate this
formula for this atom where c is the speed of light in vacuum.
c) If the electron continues to radiate at this rate how long will it take to reduce the
energy from -13.6 eV to twice this value.
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images
