.A gas cylinder (container with rigid walls) is filled with water from a high pressure supply line. Before filling, the cylinder is empty. The valve at the cylinder is then opened, exposing the cylinder to a 3.75 MPa line at 450 ℃ until the pressure in the cylinder reaches 3.75 MPa. The valve is then closed. The filling is very fast, so assume it adiabatic. (a) Determine the state of the water in the cylinder the moment the valve is closed (temperature and internal energy). (b) Determine the state of the water if the cylinder (after the valve has been closed) sits in storage at 15 ℃ for a long time. Determine the heat transfer that occurred from the moment the valve was closed until the cylinder attained the final temperature of 15 ℃. Make a comment on the sign of the heat transfer
.A gas cylinder (container with rigid walls) is filled with water from a high
pressure supply line. Before filling, the cylinder is empty. The valve at the
cylinder is then opened, exposing the cylinder to a 3.75 MPa line at 450 ℃
until the pressure in the cylinder reaches 3.75 MPa. The valve is then closed.
The filling is very fast, so assume it adiabatic.
(a) Determine the state of the water in the cylinder the moment the valve is
closed (temperature and internal energy).
(b) Determine the state of the water if the cylinder (after the valve has been
closed) sits in storage at 15 ℃ for a long time. Determine the heat transfer
that occurred from the moment the valve was closed until the cylinder
attained the final temperature of 15 ℃. Make a comment on the sign of the
heat transfer
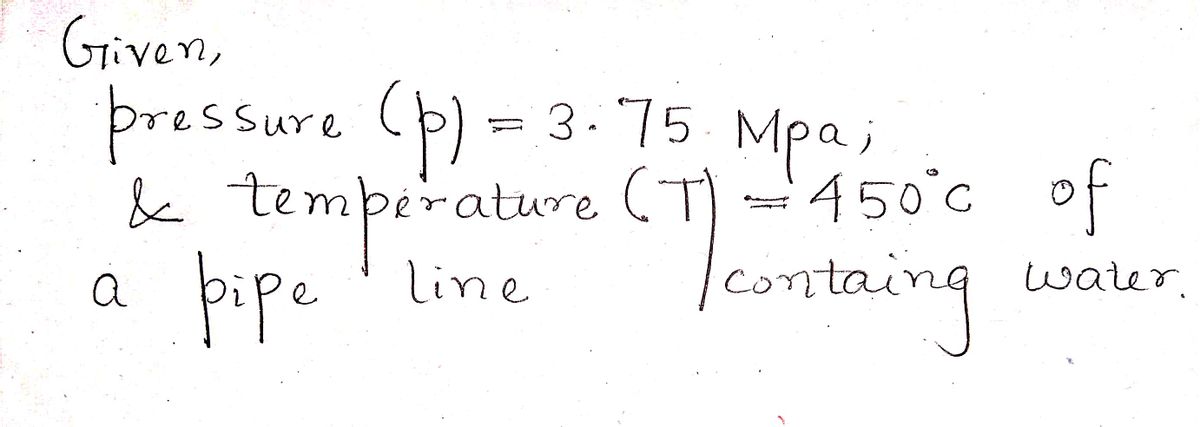
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images









