
Organic Chemistry: Principles And Mechanisms
2nd Edition
ISBN: 9780393630756
Author: KARTY, Joel
Publisher: W.w. Norton & Company,
expand_more
expand_more
format_list_bulleted
Question
Chapter G, Problem G.3YT
Interpretation Introduction
Interpretation:
Hex-1-ene undergoes ionization and fragmentation to expel a propyl radical, as shown below. The curved arrows for the fragmentation step are to be added.
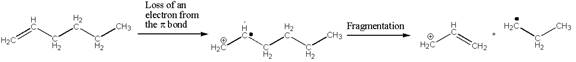
Concept introduction:
An
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculate the proton and carbon chemical shifts for this structure
A.
B.
b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion
and B. is a neutral molecule. One of these molecules is a highly reactive
compound first characterized in frozen noble gas matrices, that self-reacts
rapidly at temperatures above liquid nitrogen temperature. The other
compound was isolated at room temperature in the early 1960s, and is a
stable ligand used in organometallic chemistry. Which molecule is the more
stable molecule, and why?
Where are the chiral centers in this molecule? Also is this compound meso yes or no?
Chapter G Solutions
Organic Chemistry: Principles And Mechanisms
Knowledge Booster
Similar questions
- PLEASE HELP! URGENT!arrow_forwardWhere are the chiral centers in this molecule? Also is this compound meso yes or no?arrow_forwardA mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forward
- How many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forwardHow many chiral carbons are in the molecule? Farrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning