
Concept explainers
Practice Problem G.1
When farnesol is treated with sulfuric acid, it is converted to bisabolene. Outline a possible mechanism for this reaction.
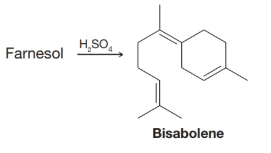
Interpretation:
The mechanism for the formation of Bisabolene from Farnesol in presence of 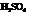 is to be outlined.
is to be outlined.
Concept introduction:
Farnesol is a compound that is formed from isoprene compounds. In general, when geranyl pyrophosphate reacts with isopenenyl pyrophosphate, farnesyl pyrophosphate (intermediate for the synthesis of sesquiterpenes) is formed. This, on oxidation can give farnesol.
Concentrated sulfuric acid acts as a strong dehydrating agent and removes the water molecule from alcohols to form unsaturated hydrocarbon.
Answer to Problem 1PP
Solution:
The mechanism for the synthesis of Bisabolene is explained as follows:

Explanation of Solution
During the first step, Farnesol (unsaturated alcohol) reacts with acid (HA = 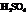 ) to form a hydrated product. Then, the hydrated product formed loses one water molecule to form a carbocation. Consequently, this carbocation rearranges to form a more stable carbocation. At last, the base
) to form a hydrated product. Then, the hydrated product formed loses one water molecule to form a carbocation. Consequently, this carbocation rearranges to form a more stable carbocation. At last, the base 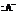 (
( 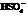 ) abstracts the hydrogen ion from the molecule, forming Bisabolene.
) abstracts the hydrogen ion from the molecule, forming Bisabolene.
The whole mechanism can be depicted as:

Hence, Bisabolene has been formed from Farnesol in the presence of concentrated sulfuric acid.
The Bisabolene has been synthesized from Farnesol in the presence of concentrated sulfuric acid. The mechanism for the synthesis of Bisabolene is outlined as:

Want to see more full solutions like this?
Chapter G Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
Additional Science Textbook Solutions
Cosmic Perspective Fundamentals
Principles of Anatomy and Physiology
Human Physiology: An Integrated Approach (8th Edition)
Chemistry: A Molecular Approach (4th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
- . Draw the products for addition reactions (label as major or minor) of the reaction between 2-methyl-2-butene and with following reactants : Steps to follow : A. These are addition reactions you need to break a double bond and make two products if possible. B. As of Markovnikov rule the hydrogen should go to that double bond carbon which has more hydrogen to make stable products or major product. Here is the link for additional help : https://study.com/academy/answer/predict-the-major-and-minor-products-of-2-methyl- 2-butene-with-hbr-as-an-electrophilic-addition-reaction-include-the-intermediate- reactions.html H₂C CH3 H H3C CH3 2-methyl-2-butene CH3 Same structure CH3 IENCESarrow_forwardDraw everything on a piece of paper including every single step and each name provided using carbons less than 3 please.arrow_forwardTopics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. H The IUPAC name isarrow_forward
- [Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardPlease draw.arrow_forwardA chromatogram with ideal Gaussian bands has tR = 9.0 minutes and w1/2 = 2.0 minutes. Find the number of theoretical plates that are present, and calculate the height of each theoretical plate if the column is 10 centimeters long.arrow_forward
- An open tubular column has an inner diameter of 207 micrometers, and the thickness of the stationary phase on the inner wall is 0.50 micrometers. Unretained solute passes through in 63 seconds and a particular solute emerges at 433 seconds. Find the distribution constant for this solute and find the fraction of time spent in the stationary phase.arrow_forwardConsider a chromatography column in which Vs= Vm/5. Find the retention factor if Kd= 3 and Kd= 30.arrow_forwardTo improve chromatographic separation, you must: Increase the number of theoretical plates on the column. Increase the height of theoretical plates on the column. Increase both the number and height of theoretical plates on the column. Increasing the flow rate of the mobile phase would Increase longitudinal diffusion Increase broadening due to mass transfer Increase broadening due to multiple paths You can improve the separation of components in gas chromatography by: Rasing the temperature of the injection port Rasing the temperature of the column isothermally Rasing the temperature of the column using temperature programming In GC, separation between two different solutes occurs because the solutes have different solubilities in the mobile phase the solutes volatilize at different rates in the injector the solutes spend different amounts of time in the stationary phasearrow_forward
- please draw and example of the following: Show the base pair connection(hydrogen bond) in DNA and RNAarrow_forwardNaming and drawing secondary Write the systematic (IUPAC) name for each of the following organic molecules: CH3 Z structure CH3 CH2 CH2 N-CH3 CH3-CH2-CH2-CH-CH3 NH CH3-CH-CH2-CH2-CH2-CH2-CH2-CH3 Explanation Check ☐ name ☐ 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C Garrow_forwardC This question shows how molecular orbital (MO) theory can be used to understand the chemical properties of elemental oxygen O₂ and its anionic derivative superoxide Oz. a) Draw the MO energy diagram for both O2 and O2. Clearly label your diagram with atomic orbital names and molecular orbital symmetry labels and include electrons. Draw the Lewis structure of O2. How does the MO description of O2 differ from the Lewis structure, and how does this difference relate to the high reactivity and magnetic properties of oxygen? ) Use the MO diagram in (a) to explain the difference in bond length and bond energy between superoxide ion (Oz, 135 pm, 360 kJ/mol) and oxygen (O2, 120.8 pm, 494 kJ/mol).arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

