
Concept explainers
(a)
Interpretation:
The structure for the given trivial name is to be drawn, and the correct IUPAC name is to be written.
Concept introduction:
The trivial names are commonly used names which are not systematic ones. Many trivial names are accepted by IUPAC. Nitriles are derivatives of
In naming organic compounds, the
Number the carbon chain in a way that the functional group and the substituents attached get the lowest number. The position of the functional group and substituents on the parent chain or ring is indicated by the respective locant number just before the suffix. Prefixes are used to denote the number of identical substituents. The substituents are written in alphabetical order when writing the IUPAC name.
Answer to Problem F.30P
The structure and IUPAC name for the given trivial name is
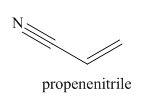
Explanation of Solution
The given trivial name is acrylonitrile.
The given name indicates that the high priority functional group is cynide. The trival name acrylonitrile correlates the derivative of acrylic acid. Structure for acrylic acid is
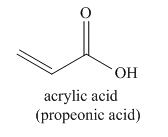
Nitriles are derived from acids where
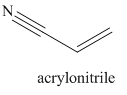
The main chain in this molecule is of three carbon atoms thus the root name is propan, and the suffix nitrile is added to the root name. The main chain is numbered starting from the carbon atom of the nitrile group. The main chain has a C=C bond; thus the IUPAC name for acrilonitrile is propenenitrile.
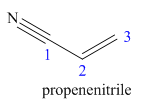
The structure for the given molecule is drawn based on the functional group, and the IUPAC named is written by adding locators to the substituents.
(b)
Interpretation:
The structure for the given trivial name is to be drawn, and the correct IUPAC name is to be written.
Concept introduction:
The trivial names are commonly used names which are not systematic ones. Many trivial names are accepted by IUPAC. Nitriles are derivatives of carboxylic acid. The trivial names of nitriles are deriving from the trivial names of the analogous carboxylic acids.
In naming organic compounds, the functional groups other than the highest priority functional groups are treated as substituents. The root name is established by identifying the longest carbon chain or a ring containing functional group. Remove the e from the normal ane, ene, or yne ending, and add the suffix that corresponds to the highest-priority functional group. Nitriles are the derivatives of carboxylic acid where
Number the carbon chain in a way that the functional group and the substituents attached get the lowest number. The position of the functional group and substituents on the parent chain or ring is indicated by the respective locant number just before the suffix. Prefixes are used to denote the number of identical substituents. The substituents are written in alphabetical order when writing the IUPAC name.
Answer to Problem F.30P
The structure and IUPAC name for the given trivial name is
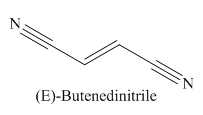
Explanation of Solution
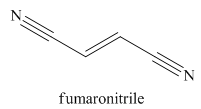
The given trivial name is fumaronitrile.
The given name indicates that the high priority functional group is cynide. The trival name fumaronitrile correlates to the derivative of fumaric acid (dicarboxylic acid). Structure for fumaric acid is
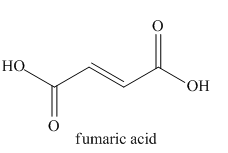
Nitriles are derived from acids where
The main chain in this molecule is of four carbon atoms; thus the root name is butan. The structure has two cynide groups at both terminals; hence the suffix dinitrile is added to the root name. The main chain is numbered starting from the carbon atom of the nitrile group.
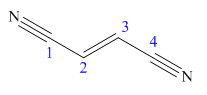
The main chain has a C=C bond; thus the IUPAC name for fumaronitrile is (E) butenedinitrile.
The structure for the given molecule is drawn based on the functional group, and the IUPAC named is written by adding locators to the substituents.
(c)
Interpretation:
The structure for the given trivial name is to be drawn, and the correct IUPAC name is to be written.
Concept introduction:
The trivial names are commonly used names which are not systematic ones. Many trivial names are accepted by IUPAC. Nitriles are derivatives of carboxylic acid. The trivial names of nitriles are deriving from the trivial names of the analogous carboxylic acids.
In naming organic compounds, the functional groups other than the highest priority functional groups are treated as substituents. The root name is established by identifying the longest carbon chain or a ring containing functional group. Remove the e from the normal ane, ene, or yne ending, and add the suffix that corresponds to the highest-priority functional group. Nitriles are the derivatives of carboxylic acid where
Number the carbon chain in a way that the functional group and the substituents attached get the lowest number. The position of the functional group and substituents on the parent chain or ring is indicated by the respective locant number just before the suffix. Prefixes are used to denote the number of identical substituents. The substituents are written in alphabetical order when writing the IUPAC name.
Answer to Problem F.30P
The structure and IUPAC name for the given trivial name is
Explanation of Solution
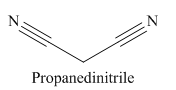
The given trivial name is malonitrile.
The given name indicates that the high priority functional group is cynide. The trival name malonitrile correlates to the derivative of malonic acid (dicarboxylic acid). Structure for malonic acid is
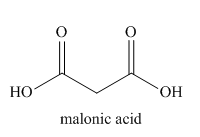
Nitriles are derived from acids where
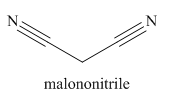
The main chain in this molecule is of three carbon atoms; thus the root name is propane. The structure has two cynide groups; hence the suffix dinitrile is added to the root name. The main chain is numbered starting from the carbon atom of nitrile group.
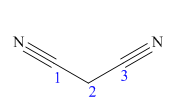
Hence the IUPAC name for malonitrile is propanedinitrile.
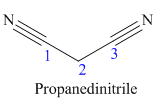
The structure for the given molecule is drawn based on the functional group, and the IUPAC named is written by adding locators to the substituents.
(d)
Interpretation:
The structure for the given trivial name is to be drawn, and the correct IUPAC name is to be written.
Concept introduction:
The trivial names are commonly used names which are not systematic ones. Many trivial names are accepted by IUPAC. Nitriles are derivatives of carboxylic acid. The trivial names of nitriles are deriving from the trivial names of the analogous carboxylic acids.
In naming organic compounds, the functional groups other than the highest priority functional groups are treated as substituents. The root name is established by identifying the longest carbon chain or a ring containing functional group. Remove the e from the normal ane, ene, or yne ending, and add the suffix that corresponds to the highest-priority functional group. Nitriles are the derivatives of carboxylic acid where
Number the carbon chain in a way that the functional group and the substituents attached get the lowest number. The position of the functional group and substituents on the parent chain or ring is indicated by the respective locant number just before the suffix. Prefixes are used to denote the number of identical substituents. The substituents are written in alphabetical order when writing the IUPAC name.
Answer to Problem F.30P
The structure and IUPAC name for the given trivial name is
Explanation of Solution
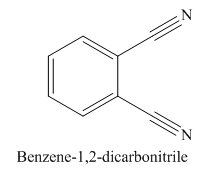
The given trivial name is phthalonitrile.
The given name indicates that the high priority functional group is cynide. The trival name phthalonitrile correlates to the derivative of phthalic acid (dicarboxylic acid). Structure for phthalic acid is
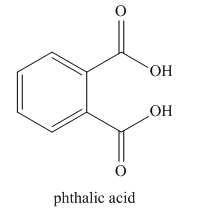
Nitriles are derived from acids where
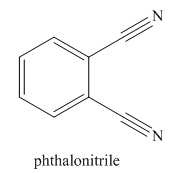
The parent ring in this molecule is benzene ring; thus the root name is benzene. The structure has two cynide groups attached directly to the ring; hence the suffix dicarbonitrile is added to the root name. The ring is numbered such that carbon atoms attached to nitrile groups get least number.
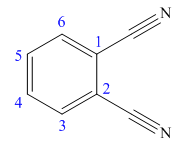
The IUPAC name for phthalonitrile is bemezene-1, 2-dicarbonitrile because two cynide groups are at C1 and C2.
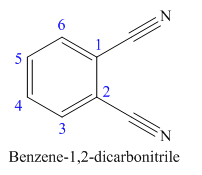
The structure for the given molecule is drawn based on the functional group, and the IUPAC named is written by adding locators to the substituents.
(e)
Interpretation:
The structure for the given trivial name is to be drawn, and the correct IUPAC name is to be written.
Concept introduction:
The trivial names are commonly used names which are not systematic ones. Many trivial names are accepted by IUPAC. Nitriles are derivatives of carboxylic acid. The trivial names of nitriles are deriving from the trivial names of the analogous carboxylic acids.
In naming organic compounds, the functional groups other than the highest priority functional groups are treated as substituents. The root name is established by identifying the longest carbon chain or a ring containing functional group. Remove the e from the normal ane, ene, or yne ending, and add the suffix that corresponds to the highest-priority functional group. Nitriles are the derivatives of carboxylic acid where
Number the carbon chain in a way that the functional group and the substituents attached get the lowest number. The position of the functional group and substituents on the parent chain or ring is indicated by the respective locant number just before the suffix. Prefixes are used to denote the number of identical substituents. The substituents are written in alphabetical order when writing the IUPAC name.
Answer to Problem F.30P
The structure and IUPAC name for the given trivial name is
Explanation of Solution
The given trivial name is valeronitrile.
The given name indicates that the high priority functional group is cynide. The trival name valeronitrile correlates to the derivative of valeric acid. Structure for valeric acid is
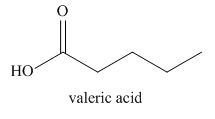
Nitriles are derived from acids where
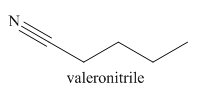
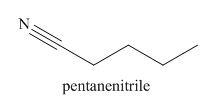
The main chain in this molecule is of five carbon atoms; thus the root name is pentane. The structure has a cynide group; hence the suffix nitrile is added to the root name. The main chain is numbered starting from the carbon atom of nitrile group.
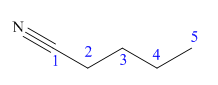
Hence the IUPAC name for valronitrile is pentanenitrile.
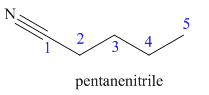
The structure for the given molecule is drawn based on the functional group, and the IUPAC named is written by adding locators to the substituents.
Want to see more full solutions like this?
Chapter F Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- What are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardWhat are the major products of the following organic reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forwardPredict the organic product that forms in the reaction below: H + гон OH H+ H+ ☑ O Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the product. In the drawing area below, draw the skeletal ("line") structure of the missing organic product X. Explanation Check Click and drag to start drawing a structure. S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forward
- In the analysis of Mg content in a 25 mL sample, a titration volume of 5 mL was obtained using 0.01 M EDTA. Calculate the Mg content in the sample if the Ca content is 20 ppmarrow_forwardPredict the organic products that form in the reaction below: H. H+ + OH H+ Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Explanation Check Click and drag to start drawing a structure. G X C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Access +arrow_forward111 Carbonyl Chem Choosing reagants for a Wittig reaction What would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 1 2 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Explanation Check Click and drag to start drawing a structure. × ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forward
- A student proposes the transformation below in one step of an organic synthesis. There may be one or more reactants missing from the left-hand side, but there are no products missing from the right-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. . If the student's transformation is possible, then complete the reaction by adding any missing reactants to the left-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + T X O O лет-ле HO OH HO OH This transformation can't be done in one step.arrow_forwardDetermine the structures of the missing organic molecules in the following reaction: X+H₂O H* H+ Y OH OH Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. X Sarrow_forwardPredict the major products of this organic reaction. If there aren't any products, because nothing will happen, check the box under the drawing area instead. No reaction. HO. O :☐ + G Na O.H Click and drag to start drawing a structure. XS xs H₂Oarrow_forward
- What are the angles a and b in the actual molecule of which this is a Lewis structure? H H C H- a -H b H Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal groups may have slightly different sizes. a = b = 0 °arrow_forwardWhat are the angles a and b in the actual molecule of which this is a Lewis structure? :0: HCOH a Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. a = 0 b=0° Sarrow_forwardDetermine the structures of the missing organic molecules in the following reaction: + H₂O +H OH O OH +H OH X Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structure of the missing organic molecule X. Click and drag to start drawing a structure.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





