
ORGANIC CHEM. VOL.1+2-W/WILEYPLUS
12th Edition
ISBN: 9781119304241
Author: Solomons
Publisher: WILEY C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter D, Problem 8PP
Practice Problem D.8
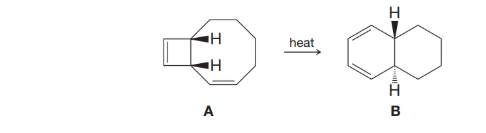
When compound A is heated, compound B can be isolated from the reaction mixture. A sequence of two electrocyclic reactions occurs; the first involves a 4πp-electron system, and the second involves a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter D Solutions
ORGANIC CHEM. VOL.1+2-W/WILEYPLUS
Ch. D - Prob. 1PPCh. D - Practice Problem D.2 (a) Show the orbitals...Ch. D - Prob. 3PPCh. D - Prob. 4PPCh. D - Prob. 5PPCh. D - Prob. 6PPCh. D - Practice Problem D.7 Can you suggest a...Ch. D - Practice Problem D.8 When compound A is heated,...Ch. D - Prob. 9PPCh. D - Practice Problem D.10 What reactant could lead to...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The Law Conversation of Mass needs to be explained. Concept introduction: Elements are the basic unit of matter...
Living By Chemistry: First Edition Textbook
1.2 Ask two of your friends (not in class) to define the terms in problem1.1.
Do their answers agee with the d...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Acetobacter is necessary for only one of the steps of vitamin C manufacture. The easiest way to accomplish this...
Microbiology: An Introduction
Why is living epithelial tissue limited to a certain thickness?
Human Anatomy & Physiology (2nd Edition)
How does a glacier change the shape and depth of a mountain valley?
Applications and Investigations in Earth Science (9th Edition)
Look at the relative positions of each pair of atoms listed here in the periodic table. How many core electrons...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License